Sustainability of Biologic Therapy is Less in Ulcerative Colitis than Crohn’s Disease Patients, Independent of Prior Biologic Experience
Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition that predominantly affects the bowel and includes both Ulcerative Colitis (UC) and Crohn’s Disease (CD). The burden of IBD is rising globally with a marked increase in the prevalence of IBD worldwide over the last 30 years [1]. The therapeutic goal in the treatment of IBD has evolved from focusing on symptomatic relief to aiming to achieve intestinal mucosal healing. Emerging longitudinal data suggests that individuals who obtain mucosal healing, which is observed more frequently with biologic than conventional therapies, have a superior long‐term prognosis with lower risk of surgery and hospitalization [2]. While there has been an expansion in the number of medications available for treating IBD including anti-tumour necrosis factor (TNF) agents, selective anti-integrins, anti-interleukins (IL) and most recently small molecule inhibitors such as the janus kinase (JAK) inhibitors, there still remains a large unmet clinical need for further therapies. Studies have shown between one third to half of patients commenced on anti-TNFs do not maintain response and require a switch to a second biologic [3]. Reasons for discontinuation vary but are often secondary to nonsustained response to a particular agent (primary non-response and secondary loss of response) or development of intolerance to a particular biologic. Chen et al looked at over 8000 patients with IBD on biologics and found after 1 year less than 50% of patients were still on this biologic agent [4].
With the evolution of medical management of IBD and new treatment goals there is a need to identify factors that predict response to biologics. To date age at diagnosis, disease duration, disease severity, and elevated inflammatory markers are all factors that have been identified to influence response to biologics [5]. However, few studies have specifically looked at real world sustainability of response to biologics, factors which might determine this and whether there is a difference between UC and CD. Given the distinct biological differences between UC and CD we aimed to look at the difference in sustainability between biologics in biologic-naïve and biologic-experienced patients depending on whether they had a diagnosis of UC or CD.
Material and Methods
Participants
A prospectively maintained electronic database of 4200 patients with IBD attending four hospitals in a single healthcare network was interrogated from January 2008 to March 2018. All patients with an established diagnosis of CD or UC currently and previously on biologic therapy were included in this analysis.
Study Design
Basic demographic information was collected including age, gender, disease subtype, age at diagnosis, type of biologic therapy used and concurrent immunomodulator therapy. Date patients commenced on a particular biologic was collected along with the date of discontinuation of biologic therapy (or last follow-up date if patients were still on this agent) due to inadequate treatment response or adverse effects. Where treatment was discontinued for other reasons (patient choice, sustained remission, or nonadherence) these treatment episodes were excluded. Whether a patient was biologic-naïve or had prior treatment with a different biologic agent (biologic-experienced) was also recorded for analysis. Ethical approval for this study was granted by the Ethics Committee at St Vincent’s University Hospital.
Outcomes
The primary outcome of interest was time to discontinuation of biologics in biologic-naïve and biologic-experienced patients’ dependent on whether patients were diagnosed of UC (UC and IBD-U) or CD. Secondary outcomes of interest included the identification of disease characteristics that impact discontinuation of biologics including immunomodulator co-therapy, age at diagnosis, disease duration, gender and disease location for both UC and CD.
Statistical Methods
Descriptive statistics were provided to summarize demographic characteristics using mean [95% confidence interval (CI)] or median [interquartile range (IQR)] for continuous variables, and number and percentage for categorical variables. Cumulative survival rates were estimated using the Kaplan-Meier method and mean and median survival time used. The log-rank test was used to compare the survival curves across the subgroups. A multivariate (adjusted analysis) Cox proportional hazards model was utilized to identify risk factors relating to survival. A two-sided P-value ≤ 0.05 was considered statistically significant. All data analyses were performed using SPSS 25.0.
Results
Baseline Characteristics
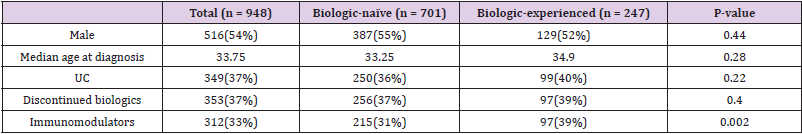
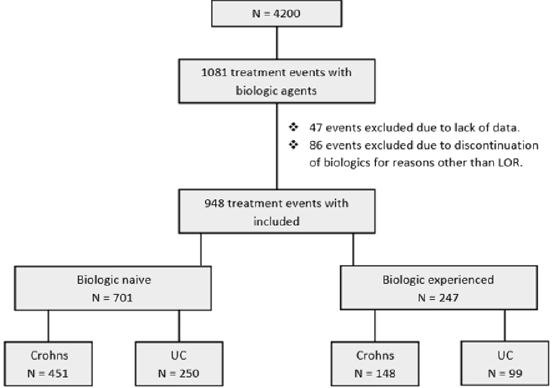
A total of 1081 treatment events with biologic therapy occurred at our centre at the time of this study. 47 events were excluded due to lack of data and 86 were excluded due to discontinuation of a biologic agent for reasons other than loss of response. In total 948 independent treatment events with biologic therapy were included in this study in 712 patients. In total 516 (54.4 %) patients were male. Median age at diagnosis was 33.8 years (IQR 24.6 – 44.8). 349 (36.8 %) patients had a diagnosis of UC. 353 (37.2 %) patients in total discontinued their biologic agents. 312 (32.9 %) patients were on co-therapy with immunomodulators. Mean follow-up was 2.3 years (IQR 0.64– 3.2) (Table 1). Patients were sub-divided depending on whether they were biologicnaïve (Group1) or biologic experienced (Group 2) (Figure 1).
Sustainability of Biologics Between UC and CD
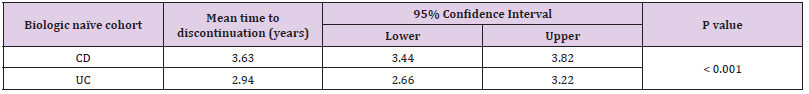
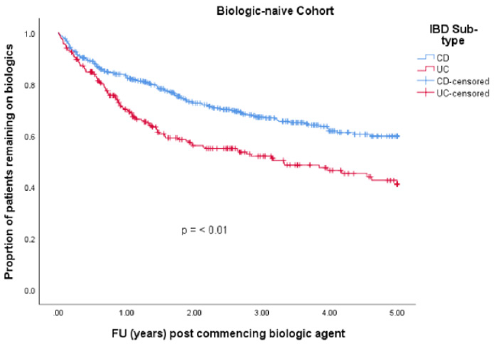
• Group 1: 701 (74 %) patients were biologic-naïve. 387 (55.2 %) were male. Median age at diagnosis was 33.3 years (IQR 23.7 – 44.5). 250 (35.7 %) had a diagnosis of UC and 451 (64.3 %) had CD. Median disease duration was 7.5 years (IQR 3.1 – 13.9). 256 (36.5 %) patients discontinued their biologics. 215 (30.7 %) patients were on co-therapy with immunomodulators (Table 1). 329 (46.9 %) patients received infliximab (IFX), 337 (48.1 %) adalimumab (ADA), 32 (4.6 %) golimumab (GLM) and 3 (0.4 %) received ustekinumab (UST). Mean time to discontinuation of biologics in the UC cohort was 2.9 years (95% CI 2.6 – 3.22 years) which was significantly shorter than in the CD cohort which was 3.6 years (95% CI 3.4 – 3.8 years) (p-value = < 0.001) (Table 2 & Figure 2).
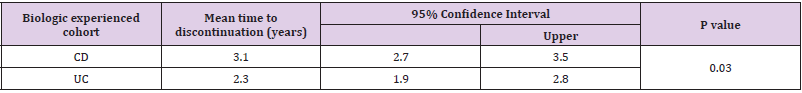
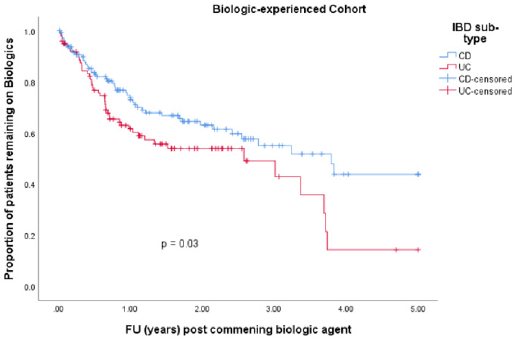
• Group 2: 247 (26.1 %) patients were biologic experienced. 129 (52.2 %) were male. Median age at diagnosis was 34.9 years (IQR 27.8 – 45.0). 99 (40.1 %) had a diagnosis of UC and 148 (59.9 %) had CD. Median disease duration was 10.5 years (IQR 5.2 – 15.9). 97 (39.3 %) patients discontinued their biologics. 97 (39.3 %) patients were on co-therapy with immunomodulators (Table 1). 83 (33.6 %) patients received IFX, 57 (23.1 %) ADA, 40 (16.2 %) vedolizumab, 26 (10.5 %) GLM and 41 (16.6%) received UST. Mean time to discontinuation of biologics in the CD cohort was 3.1 years (95% CI 2.7 – 3.5 years) compared with 2.3 years (95% CI 1.9 – 2.8 years) in the UC cohort (p-value = 0.030) (Table 3 & Figure 3).
Factors Affecting Discontinuation of Biologics
We looked at gender, age at diagnosis, co-therapy with immunomodulators, IBD subtype and disease duration as potential factors impacting biologic discontinuation.
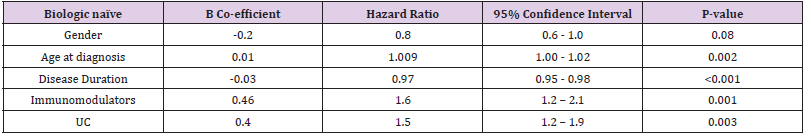
• Group 1: Gender had no impact on discontinuation of biologic agents with a hazard ratio (HR) 0.8 (p-value 0.07, 95% CI 0.6-1.0). Older age at diagnosis was associated with a slight increased risk of discontinuing a biologic agent with a HR 1.009 (p-value 0.002, 95% CI 1.00-1.02). Shorter disease duration was protective against discontinuation of biologics with a HR of 0.97 (p-value <0.001, 95% CI 0.95 – 0.98). Co-therapy with immunomodulators increased the risk of discontinuing biologic therapy in a biologic-naïve cohort with a HR 1.6 (p-value 0.001, 95% CI 1.2 – 2.1). UC as seen in our survival analysis increased your risk of discontinuing biologic therapy in our biologic naïve cohort with a HR 1.5 (p-value 0.003, 95% CI 1.2 – 1.9) (Table 4).
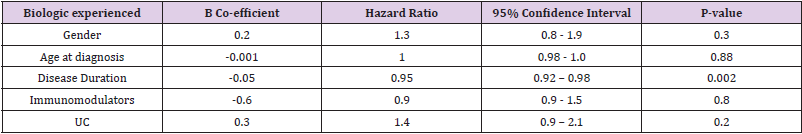
• Group 2: Neither gender nor age at diagnosis showed any independent association with time to discontinuation of biologics in our biologic-experienced cohort. Shorter disease duration was again protective against discontinuation of biologics with a HR of 0.95 (p-value 0.002, 95% CI 0.92-0.98). Co-therapy with immunomodulator therapy had no significant impact on discontinuation of biologics in our biologic-experienced cohort with a HR 0.9 (p-value 0.8, 95% CI 0.9 – 1.5) and neither did a diagnosis of UC (p-value 0.2, 95% CI 0.9 – 2.1) (Table 5).
Discussion
Over the past two decades there has been significant advances in the medical management of IBD especially since the introduction of biologic agents. Unfortunately, despite current advances loss of response to biologics still affects up to 30-40% of patients in clinical practise [5]. Identifying factors that predict the efficacy of biologics is important to efficiently use these agents along with minimising side-effects and improving cost-effectiveness. To date certain disease related factors which impact response to anti-TNF agents include gender, age at diagnosis or the presence of fistulating disease [5,6] but overall data is limited. Interestingly, despite the anatomical, genetic, and immunological differences between CD and UC there is no evidence to date indicating biologics work more effectively in either sub-type of IBD. Our study is the first to investigate the impact of IBD subtype on sustainability of biologics and our findings show that in both biologic-naïve and biologic-experienced patients’ biologic therapy is more durable in CD than UC. In our biologic-naïve cohort patients with CD were significantly less likely to discontinue a biologic agent compared with UC and similar results were seen in patients who were biologicexperienced. More research is required to identify unique reasons why biologic therapy appears more sustainable in CD but one can hypothesis it is most likely multi-factorial. Certain disease related differences between CD and UC including genetic, immunogenic and differences in the gut microbiota can impact response to treatment.
Other modifiable factors such as differences in the inflammatory burden between CD and UC and how each physician manages and adjusts their patient’s treatment can certainly influence response to treatments. In CD, it is well known that surgery is not curative, and it is standard practice to favor bowel-sparing surgical approaches, and resection of the affected part of bowel only is preferable [7]. Interestingly after the introduction of anti-TNF therapy the risk for IBD related hospitalization have only decreased in CD but not in UC [8]. Unlike CD with UC surgery is often considered curative and this can impact both physician and patient decision making and treatment goals. From recent studies there is also a clear difference in the prescribing practise of biologics between UC and CD. One cross-sectional study analysing United States (US) health care utilization in 964,633 IBD patients between 2010 and 2012 showed that UC patients were much less frequently treated with biologics than CD patients (3.5% vs 16.8%) [9]. A second study in the US of over 400,000 IBD patients showed that the proportion of patients using biologics increased for both UC and CD between 2007 to 2015, but to a much higher extent in patients with CD (from 21.8% to 43.8%) than in patients with UC (from 5.1% to 16.2%) [10]. Numerous disease related factors impact response to biologic therapy other than the sub-type of IBD. In our study shorter disease durations and older age at diagnosis had a negative impact on sustainability of biologics.
Regarding disease duration studies are conflicting. Several studies have shown in CD shorter disease duration improves response to biologics [11-13] however the opposite has been in seen in two recent studies, one looking at anti-TNF therapy and one looking at vedolizumab response [14,15]. In our study, like Nguyen et al’s study patients with a shorter disease duration were more likely to discontinue biologic agents sooner. In our study older age at diagnosis was associated with a slightly increased risk of discontinuing biologic agents in our biologic-naïve cohort. Desai et al showed similar results in a small study looking where an age over 60 years correlated with an increased risk of discontinuation of biologics [16]. Co-therapy with immunomodulators is advised by ECCO guidelines for management of patients with moderate to severe IBD treated with IFX to help achieve and maintain remission however not for adalimumab [17]. Our study found in our biologic-naïve cohort co-therapy with immunomodulators had a negative impact on sustainability of biologics with a HR 1.6 which is expected given 48% of our biologic-naïve cohort were treated with adalimumab and 47% with IFX. In our study 37% of patients discontinued a particular biologic agent over the 5-year follow-up. The incidence of primary nonresponse to anti-TNFs varies from 10 to 30% in clinical trials and clinical practise [18-20].
The annual risk of secondary loss of response in one particular review for IFX was 13% per patient year [21] and 20.3% for adalimumab in two separate study [22]. This data highlights the need for a personalised approach to treatment for each patient with IBD. Promising targets for new therapeutic strategies are currently being developed and our study highlights the need to broaden treatment options available for IBD to ensure patients both achieve and sustain mucosal healing given the chronic nature of IBD. Limitations of our study include the retrospective and observational nature without randomization or blinding. Secondly data was not collected on disease severity at onset of biologics which can impact response to treatment. Another limitation is the variability in prescribing biologics and escalation of treatment between different gastroenterologists at our centre.
Conclusion
In conclusion our real word data indicates sustainability of biologic therapies is less in UC than CD and this is not strongly determined by prior biologic exposure. The reason biologics are more sustainable in CD is most likely multifactorial however this finding is important in determining how biologic agents are employed in both IBD subtypes. Our data also highlights the need for new biologics and small molecules inhibitors as there is currently an unmet medical need for treatments in UC and CD given the high rates of primary and secondary loss of response.
For more Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.