Marine Algal Bioactive Metabolites: Effects and Occurrence
Mini Review
When the algal density reached over the baseline level and caused harmful effects, these algal blooms are defined as harmful algal blooms (HABs) Hallegraeff [1]. Over the past several decades, marine algal bioactive metabolites have become a concern for the environment and human health. The contact (e.g., ingestion) of these metabolites results in an alternation of cellular enzyme functionality and causes cell deformation and mortality in the worst cases. Marine diatoms, dinoflagellates, and cyanobacteria are the known producers of these harmful metabolites. Bioactivity and the occurrence of these algal metabolites will be reviewed herein. Marine diatoms were the known producer of domoic acid. In 1987, ingestion of cultured blue mussels (Mytilus edulis) containing domoic acid (DA) caused food poisoning that killed three people and sickened >100 others Bates, et al. [2,3]. The structure of DA was determined to be an analogue of glutamic acid (Wright et al., 1989), and known producers of DA are species of a marine diatom genus Pseudo-nitzschia Jeffery, et al. [4]. DA is a neurotoxin that causes neuronal degeneration and necrosis in specific hippocampus regions, leading to amnesic shellfish poisoning. Several reports on the accumulation of DA in various organisms. However, DA can be degraded through the process of frozen storage and cooking, suggesting the low stability of this compound (as cited in Jeffery, et al. [4]).
Marine dinoflagellates are known producers of a series of bioactive metabolites that are classified into five major groups by their bioactivities after ingestion of toxin-containing fish and shellfish. These bioactivities including paralytic shellfish poisoning (PSP), diarrhoeic shellfish poisoning (DSP), neurologic shellfish poisoning (NSP), azaspiracid shellfish poisoning (AZP), and ciguatera fish poisoning (CFP) metabolites. PSP in humans is caused by the ingestion of seafood containing a group of alkaloids, including saxitoxin and its analogues (Cusick, et al. [5]). The pharmacological action of the PSP toxin is characterized as the blockage of the voltage-gated sodium channel (VGSC), leading to numbness and respiratory paralysis that could be fatal. Marine dinoflagellates (e.g., Alexandrium) and freshwater cyanobacteria (e.g., Dolichospermum) are the known producers of PSP toxins. Diarrhea, nausea, vomiting, liver necrosis, cardiac muscle damage, and abdominal pain are the know symptoms of DSP, caused by the ingestion of bivalves containing lipophilic metabolites, including okadaic acid (OA) and dinophysistoxins (DTX) (Reguera, et al. [6]). Marine species of Dinophysis and Prorocentrum are the and pectenotoxins (PTX) are two groups of lipophilic toxins that are structurally distinct from OA and DTX but possess similar bioactivities. However, some YTX and PTX can cause liver necrosis and cardiac muscle damage without diarrhea (Domínguez, et al. [7]). The common origins of YTX are marine species of Prorocentrum, and PTX are produced by Dinophysis.
The Florida and Gulf of Mexico coastal red tide former, Karenia brevis (syn. Gymnodinium breve and Ptychodiscus breve), is the common producer of lipophilic brevetoxins (PbTX) that cause NSP (Baden, et al. [8]). Structurally, PbTX can be divided into two groups, one with 10 cyclic rings and the other with 11 rings. The toxicity of NSP toxins was caused by opening the VGSC, leading to nausea, vomiting, paralysis, seizures, and coma. The aerosolization of PbTX caused by wave action leads to asthmalike symptoms in humans. The next dinoflagellate toxin group is AZP toxins, including azaspiracids (AZA). To date, over 50 AZA were isolated from species in marine dinoflagellate Azadinium, and contaminated seafood (Twiner, et al. [9,10]). The ingestion of AZA caused nausea, vomiting, diarrhea, and stomach cramps (Twiner, et al. [9,10]), while the mechanism of action has not been elucidated. The final dinoflagellate toxin group is CFP that caused by lipophilic ciguatoxins (CTX). The common origin of CTX is Gambierdiscus toxicus, while species in Prorocentrum were also reported as producers (Friedman, et al. [11]). CTX found in the Pacific region (n=13) had a different number of cyclic rings than CTX in the Caribbean region (n=14). CTX mechanism of action is similar to PbTX; however, some CTX had a greater affinity for VGSC than PbTX. Cyanobacteria are prolific bioactive secondary metabolite producers. To date, 157 known bioactive classes have been identified. Four (i.e., microcystins, saxitoxin, anatoxin-a, and cylindrospermopsins) of these known bioactive classes were listed in the EPA Contaminant Candidate List 4 (CCL4).
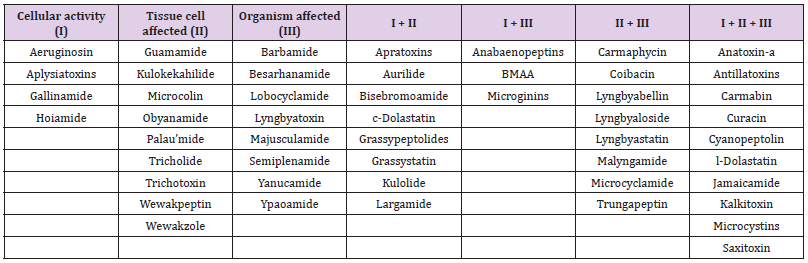
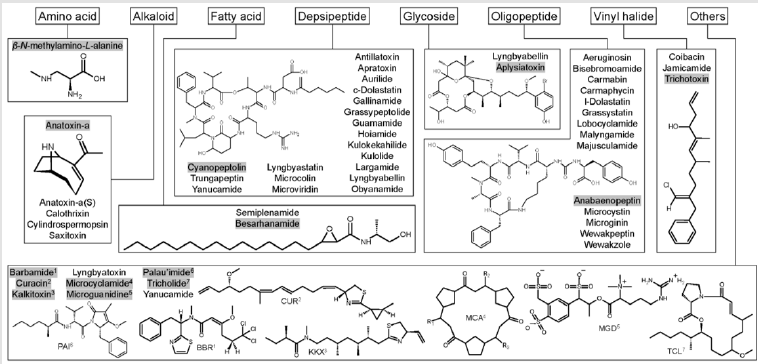
In the author’s previous effort, the 157 known bioactive classes have been reclassified to 55 structurally unique bioactive classes based on similarities of their structure and biological activity (Huang, et al. [12]). This effort was necessary because some metabolites share similar chemical structures and bioactivity but have been named differently. Therefore, a classification system was proposed to include both the original class names and reclassified class names. For example, lyngbyaureidamides have a similar structure as anabaenopeptins. Thus, when describing this compound, the proposed description will be anabaenopeptinlyngbyaureidamides. Fifty of 55 classes, including isomers/ synonyms, have been described from the marine environment (Table 1). Three of the four EPA CCL4 listed classes have been described from marine cyanobacteria: microcystins, anatoxin-a, and saxitoxin. Thirty of these 55 marine secondary groups were originally isolated from benthic cyanobacteria (e.g., Lyngbya and Moorea), with some compounds were elucidated from marine invertebrates (e.g., Dolabella). Over the past three decades, the knowledge of marine cyanobacterial metabolites has increased tremendously (Huang, et al. [13-15]). Their structures belong to eight main groups: amino acids, alkaloids, fatty acids, depsipeptides, glycosides, oligopeptides, vinyl halides, and other structures (Figure 1). Some cyanobacterial bioactive metabolites are chlorinated and brominated. The bioactivity of these compounds started from enzyme inhibition and VGSC blockage, causing organ bleeding and swelling, eventually, the death of the organisms (Table 1).
Table 1: Summary of marine cyanobacterial metabolites bioactivities. The column with “I + II”, “I + III”, “II + III”, and “I + II + III” presented a combination of bioactivity has been found. For example, compounds in column “I + II” contain both cellular and tissue cell activity.
Figure 1:Summary of cyanobacterial bioactive compound general structures. These compounds can be classified into eight groups: amino acids, alkaloids, fatty acids, depsipeptides, glycosides, oligopeptides, vinyl halides, and other structures. In addition, the structures of gray-highlighted compounds are shown. Among these classes, anatoxin-a(S), calothrixins, microguanidines, microviridins, and cylindrospermopsins have not been reported in the marine ecosystem.
Algal blooms will likely increase in frequency and intensity due to climate change and anthropogenic nutrient input. Accompanied with this trend, the frequency of toxin-producing algae cooccurrence will also be increased. Unfortunately, most researchers and monitoring programs only focus on certain toxins of interest, while other toxin classes with similar or higher toxicity are unstudied. Therefore, if two toxins carry similar toxicity co-exist, the estimate of the bloom might toxicity not be accurate. Further, HAB co-occurrence could result in synergistic effects caused by multiple bioactive metabolites that possess different known bioactivities. For example, when an enzyme inhibitor co-occurs with a linear cytotoxic oligopeptide, this inhibitor can deactivate the enzyme digesting ability on the oligopeptide, leading to more damage caused by the oligopeptide. Thus, a reevaluation of the monitoring protocols to include strategies for toxin co-occurrence is needed.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.