Disorders of Energy Metabolism in Neurons of the Cerebral Cortex During Cerebral Ischemia
Introduction
In cerebral ischemia (CI), a chain of pathogenetic disorders develops in its structures, among which one of the leading is energy deficiency, which leads to the development of cellular pathology [1,2]. The work of enzymes, including sodium-potassium ATPase, is disrupted, leading to an imbalance of ions and cerebral edema [3-6]. A number of molecular markers of the energy activity of mitochondria are known, among which one of the main markers is ATP synthase - an integral protein of the inner mitochondrial membrane that carries out the reaction of ATP formation from ADP [7,8]. Mitochondrial ATP synthase plays an important role in the differentiation of stem cells, promotes the formation of mitochondrial cristae by dimerization and specific regulation [9- 11]. The enzyme belongs to the alpha / beta ATP synthase family. Consists of two structural domains (F1 - extramembrane catalyst and F0 - membrane proton channel), connected by a central rod consisting of γ, δ and ε subunits [12-15], and together with the oligomer of the membrane subunit representing the rotary domain of the enzyme [1,4,7,16,17].Elucidation of the mechanisms of the development of energy deficiency in ischemic damage of varying severity is advisable for detailing the pathogenesis, the ratio of damage and compensation processes in СШ. The aim of the study was to assess changes in the content of ATP synthase in the parietal cortex and hippocampus of the brain of rats with ischemia of varying severity in a comparative aspect. For this purpose, the changes in the content of ATP synthase in rats with partial, subtotal, stepwise subtotal and total СI were studied [3].
Methods
The experiments were carried out on 88 male outbred white rats weighing 260 ± 20 g in compliance with the Directive of the European Parliament and of the Council No. 2010/63/EU of 22.09.2010 on the protection of animals used for scientific purposes.CI was modeled under conditions of intravenous thiopental anesthesia (40-50 mg/ kg). Total cerebral ischemia (TCI) was modeled by decapitation of animals. The brain sampling was carried out 1 hour and 24 hours after decapitation to study tissue respiration of mitochondria, and also after 1 hour to determine the content of ATP synthase. Subtotal cerebral ischemia (SCI) was modeled by simultaneous ligation of both common carotid arteries (CCA). The material was taken after 1 hour to determine the content of ATP synthase. Graduated subtotal CI (GSCI) was performed by sequential ligation of both CCA with an interval of 7 days (subgroup 1), 3 days (subgroup 2), or 1 day (subgroup 3). The sampling of material was carried out 1 hour after ligation of the second CCA in each of the subgroups. Partial cerebral ischemia (PCI) was modeled by ligating one CCA on the right. The sampling of material was carried out 1 hour after the operation [3,18].
Determination of the content of ATP synthase was carried out by immunohistochemical method using monoclonal antibodies. For this purpose, after decapitation, the brain was quickly removed from the rats, pieces of the cerebral cortex were fixed in zincethanol- formaldehyde at + 4°C (overnight), then embedded in paraffin. Paraffin sections with a thickness of 5 μm were prepared using a microtome and mounted on glass slides. The preparations were processed according to the protocol of immunocytochemical reaction for light microscopy, excluding the procedure of thermal unmasking of antigens. To determine the immunoreactivity of the molecular marker of mitochondria ATP synthase (complex V that forms ATP from ADP), primary monoclonal mouse antibodies (Anti- ATP5A antibody, Abcam, UK, ab. 14748) were used at a dilution of 1: 2400 at + 4°C, with an exposure of 20 h in a humid chamber. To detect bound primary antibodies, an EXPOSE Mouse and Rabbit specific HRP/DAB detection IHC kit Abcam (Great Britain, ab. 80436) was used. The immunoreactivity of ATP synthase was studied in the cytoplasm of neurons of the fifth layer of the parietal cortex and neurons of the CA1 field of the hippocampus in immunohistochemical preparations based on the optical density of the chromogen sediment using an Axioscop 2 plus microscope (Zeiss, Germany), a digital video camera (Leica DFC 320, Germany) and an image analysis program ImageWarp (Bitflow, USA).To prevent systematic measurement errors, brain samples from the compared control and experimental groups of animals were studied under the same conditions.
As a result of research, quantitative continuous data were obtained. In connection with the use of small samples with an abnormal distribution in the experiment, the processing was carried out by the methods of nonparametric statistics using the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). Data are presented as Me (LQ; UQ), where Me is the median, LQ is the value of the lower quartile; UQ is the upper quartile value. Differences between groups were considered significant at p <0.05 (Kruskell-Wallis test with Bonferoni’s correction) [19].
Results
In TCI, a decrease in the content of ATP synthase, which characterizes the state of the V-complex of the electron transport chain, was noted in comparison with its level in the control group - by 74 (66; 86) % in the parietal cortex, p<0.05 and by 70 (54; 81) % - in the hippocampus, p <0.05 (Table 1, Figures 1 & 2). At the same time, there were no differences in the content of the enzyme in the parietal cortex and hippocampus (p>0.05).Compared with the value in the “control”, in the SCI group, the content of ATP synthase decreased by 45 (38; 53)% - in the parietal cortex (p <0.05) and by 39 (31; 46)% - in the hippocampus (p<0.05).Compared to the level of the value in the “TCI” group, the content of ATP synthase in rats with SCI was higher in the parietal cortex by 57 (46; 61)%, p<0.05 and by 38 (32; 43)% - in the hippocampus, p<0.05.The decrease in the content of ATP synthase in rats with SIGM was less pronounced than in rats with TCI - by 29% in the parietal cortex (p <0.05) and by 39% in the hippocampus (p <0.05).With PCI, there was a decrease in the content of ATP synthase, compared with the value in the control group - in the parietal cortex by 21 (13; 32)%, p <0.05, in the hippocampus by 8 (4; 13)%, p <0 , 05.
Table 1: The content of ATP synthase in the cytoplasm of neurons in the parietal cortex and field CA1 of the hippocampus of rats with total, subtotal and partial cerebralsischemia,sMes(LQ;sUQ).
Note: * - p <0.05 compared with the “control” group, # - p<0.05 compared with the “TCI” group, + - p<0.05 compared with the “SCI” group, α - p<0.05 compared with, compared with the 1st subgroup of SCI,
β - p<0.05 in comparison with the 2nd subgroup of GSCI. TCI - total cerebral ischemia, SCI - subtotal cerebral ischemia, PCI - partial cerebral ischemia, SSCI - stepwise subtotal cerebral ischemia, sg – subgroup
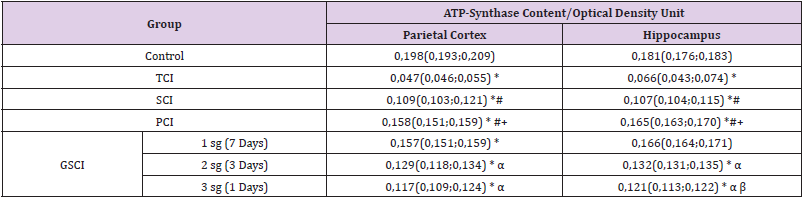
Figure 1: The content of ATP synthase in the cytoplasm of pyramidal neurons in the parietal cortex of rats with total (TCI), subtotal (SCI), stepwise subtotal (GSCI) and partial cerebral ischemia (PCI). Digital micrograph. Magnification x 40.
Note:
• Subgroup 1 - the interval between CCA dressings 7 days
• Subgroup 2 - the interval between CCA dressings is 3 days
• Subgroup 3 - the interval between CCA dressings 1 day
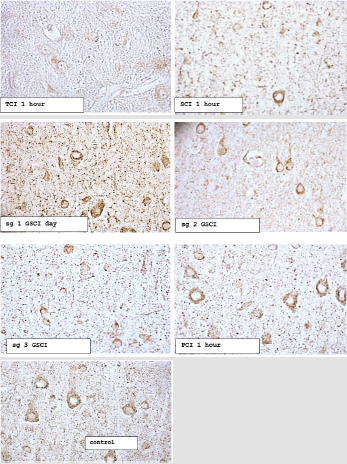
Figure 2: The content of ATP synthase in the cytoplasm of pyramidal neurons of the CA1 field of the hippocampus of rats with total (TCI), subtotal (SCI), stepwise subtotal (GSCI) and partial cerebral ischemia (PCI). Digital micrograph. Magnification x 40
Note:
subgroup 1 - the interval between CCA dressings 7 days
subgroup 2 - the interval between CCA dressings is 3 days
subgroup 3 - the interval between CCA dressings 1 day
At the same time, compared with the value in the “TIGM” group, the content of ATP synthase in the cytoplasm of neurons was 70 (66; 85)% higher in the parietal cortex (p <0.05) and by 58 (41; 76)% - in the hippocampus (p <0.05), and compared with the value in the “SCI” group - by 28 (19; 38)%, p <0.05 and 35 (27; 42)%, p <0.05 , respectively. A decrease in the ATP synthase content in the parietal cortex and hippocampus with PCI is less pronounced than with SIGM by 24% (p <0.05) and 31% (p <0.05), respectively, and than with TCI - by 53% (p <0.05) and 62% (p <0.05), respectively. Compared to the level of ATP synthase in the control group, in the 1st subgroup of SCI (the interval between dressings is 7 days), there was a decrease in the content of ATP synthase by 23 (12; 37)% in the parietal cortex (p <0 , 05) and by 7 (3; 12)% - in the hippocampus (p <0.05), in the 2nd subgroup of GSCI (the interval between dressings is 3 days), the content of ATP synthase decreased to a greater extent, amounting to 37 (27; 44)% - in the parietal cortex (p <0.05) and 26 (20; 34)% - in the hippocampus (p <0.05), and in the 3rd subgroup (the interval between dressings 1 day) there was the most significant decrease enzyme content - by 42 (35; 52)%. p <0.05 and by 33 (27; 42) %, p <0.05, respectively.
In the 3rd subgroup of GSCI (the interval between CCA dressings was 1 day), the enzyme content was less, compared with the 1st subgroup, by 27 (22; 32)% - in the parietal cortex (p <0.05) and by 18 (11 ; 23)% - in the hippocampus (p <0.05), and compared with the 2nd subgroup of GSCI - by 8 (3; 15)% in the hippocampus (p <0.05), while in the parietal cortex there were no differences was noted (p> 0.05).Thus, in the “GSCI” group, the smallest decrease in the content of ATP synthase was observed in the 1st subgroup of GSCI with an interval between dressings of 7 days, while the greatest decrease in the enzyme content was noted in the 3rd subgroup with a minimum interval between CCA dressings (1 day).
Discussion
Compared with the value in the “SCI” group, modeled by onestage ligation of both CCA, in the 3rd subgroup of SCI in both studied departments, no differences were found (p> 0.05). The content of ATP synthase in the 2nd subgroup of SCI was 17% higher than at SCI (p <0.05) in the hippocampus, while in the parietal cortex there were no differences (p> 0.05), and in 1 -th subgroup SCI - it was 30% more in the parietal cortex (p <0.05) and 34% - in the hippocampus (p <0.05) than in rats with SCI. Compared with the value in the group “PCI”, in the 1st subgroup of GSCI in both studied departments there were no differences in the content of ATP synthase (p> 0.05), in the 2nd subgroup of GSCI it was less by 18% in the parietal cortex (p <0.05) and by 20% - in the hippocampus (p <0.05), and in the 3rd subgroup of SIGM the differences from SCI were more pronounced and amounted to 26% (p <0.05) and 27% ( p <0.05), respectively. The severity of changes in the content of ATPsynthase in the 2nd and 3rd subgroups of GSCI was greater: by 18% (p <0.05) - in the hippocampus of the brain of rats of the second subgroup of SSHM and by 21% - in the parietal cortex (p <0,05) and 25% in the hippocampus (p <0.05) in the 3rd subgroup of rats with SCI. The content of ATP-synthase in the 1st subgroup of SSIGM (the interval between CCA dressings was 7 days) was the closest to the indicators in the “PCI” group, while in the 3rd subgroup of GSCI, with a minimum interval between CCA dressings of 1 day, in to a greater extent was close to the values of the enzyme content in the “SCI” group, modeled by simultaneous ligation of both CCA, which indicates a more pronounced damage to the V complex of the electron transport chain in this model of SCI.
Studies have shown the dependence of the severity of brain damage in SSHM on the interval between the cessation of blood flow in both CCA. With a 7-day interval between CCA dressings, the content of ATP synthase was restored. The results obtained agree with the previously obtained data. According to which the smallest morphological changes in neurons were noted in the “CHIGM” groups and the 1st subgroup “GSCI”, with an interval between CCA dressings of 7 days. It is obvious that with these methods of modeling IHM, adaptation processes occur that prevent the development of pronounced morphological changes and allow neurons to adapt to conditions of moderate hypoxia. So, for example, in PCI, the absence of pronounced morphological changes in rats is explained by the compensation of blood circulation in the circle of Willis. With GSCI, when the time interval (7 days between dressings) is sufficient for the development of adaptive processes, the productivity of mitochondrial respiration increases [5], and, possibly, the production of nitrogen monoxide and hypoxia-induced factor are activated [4]. Modeling of more severe types of ischemic damage leads to pronounced morphological changes in neurons in the parietal cortex and hippocampus of the rat brain - a decrease in their size, deformation of perikarya, an increase in the degree of neuronal chromatophilia with their simultaneous wrinkling and subsequent death. To the greatest extent, these disorders were expressed in the 3rd subgroup of SCI with the shortest interval between dressings, which was 1 day, and in the TCI group [4]. This, probably, can explain, in comparison with the groups “TCI”, “SCI” and the 3rd subgroup “SCI”, the higher content of ATP synthase in the cytoplasm of neurons in the 1st and 2nd subgroups “SCI” and “ PCI “.
Conclusion
Thus, the most pronounced decrease in the content of ATP synthase was observed in the groups “TCI”, “SCI” and in the 3rd subgroup “SCI”, with a minimum time interval between CCA dressings. With GSCI with an interval between CCA dressings of 7 days, the suppression of the content of ATP synthase was not so significant [20,21].



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.