Successful Treatment of a Chronically Infected and Occluded Aorto-Bifemoral Dacron® Bypass with Bacteriophages
Background
In vascular surgery, infections of the vascular grafts are considered to be severe complications [1]. Especially infections of aortic grafts are associated with a high morbidity and mortality of up to 75 % [2]. Since these procedures are often performed in patients with multiple comorbidities, the required explantation of the infected graft and the extensive struggle with the related abdominal infection is related with an early postoperative morbidity and mortality of even over 20 % [3]. Despite the initial achievement of a successful treatment, the general rate of reinfection can be up to 20 % of cases [4]. This is mainly due to bacterial colonies embedded in the peri-prosthetic tissue, which then form a surface-adherent biofilm and hence have an up to 1000-fold greater resistance to antibiotic administration [5]. Even a targeted antibiosis appropriate to antimicrobial susceptibility testing can only suppress a graft infection but does not constitute a curative treatment option [6]. The most common pathogenic bacteria associated with graft inflammation are Staphylococcus aureus, Staphylococcus epidermidis and other coagulase-negative staphylococci, Enterobacterales, Pseudomonas aeruginosa and corynebacteria [7]. These bacteria regularly enhance their specific virulence by attaching to the prosthetic material, and hence averting the local immune response by forming biofilms, that hinder phagocytosis. Furthermore, systemic antibiotic therapy is often inadequate due to the lack of effective saturation concentrations within the inflammatory periprosthetic tissue. In order to reduce the morbidity and mortality associated with the often inevitable surgical treatment, less invasive approaches to adequately treat the infection of the surrounding tissue are urgently needed. In this context, bacteriophages and their bacteriolytic activity are a promising therapeutic option.
Case
In November 2020, a 66 year-old male patient was referred to the emergency ward by his general practitioner with the clinical symptom of an acute abdomen. The examination revealed ubiquitous tenderness on all quadrants with peritonism in the lower abdomen. An infection with SARS-CoV-2 was ruled out. Further examination showed an elevated body temperature of 39.2 °C, and blood testing revealed a leukocyte count of 9.4 x 109/l, as well as an elevated serum C-reactive protein of 90.2 mg/l. The chest X-ray depicted no evidence of pneumonia. An endocarditis was ruled out. Calculated antibiotic therapy with ampicillin/sulbactam was started in the usual dosage intravenously. Blood cultures were positive for Methicillin-susceptible Staphylococcus aureus. Secondary findings included the status of ubiquitous arterial occlusive disease. Due to the necessity of numerous vascular operations on both legs, the patient had eventually undergone a thigh amputation on the right side 12 months earlier; the left side revealed a chronically occluded polytetrafluorethylen (PTFE) Stockmann bypass still in situ.
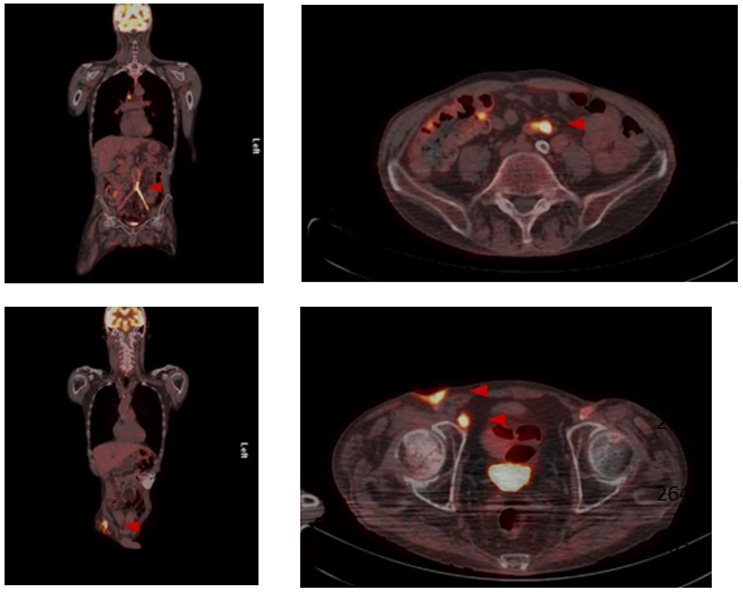
After various transfemoral surgical recanalization attempts in the anamnesis, there were hostile tissue conditions bifemoral with a chronic wound infection leading to exposed graft material. Wound swabs exposed the presence of Staphylococcus aureus and Escherichia coli, indicating a polymicrobial infection. The peripheral blood flow of the lower limbs was compensated. Initially, a CT scan of the abdomen was performed, whereupon an occluded and infected aorto-bifemoral graft was assumed. The subsequently performed PET- CT scan displayed a visibly increased metabolic activity in the area of the graft, so that we diagnosed a chronically occluded and infected aorto-bifemoral prosthetic bypass with subsequential bifemoral infections, leading to the cutaneous wound healing disorder (Figure 1). Due to the patient’s comorbidities, we generally intended an operation and anesthesia time as short as possible with an efficacious treatment by explanting the prosthetic bypass. Further we planned to forego a lavage program for the septic abdomen and intended a primary closure of the abdomen.
In order to treat the local inflammation in the abdominal and femoral areas in the long term intra- and postoperatively, the use of bacteriophages was considered to be plausible alternative therapy option in this case. The patient himself favored an alternative solution compared to an indefinite lasting systemical antibacterial treatment. Therefore, an experimental approach using local bacteriophage application was intended as a last resort treatment in line with Article 37 of the Declaration of Helsinki and in unity with the local ethics committee (A 2021-0208).
Figure 1: Preoperative PET-CT.
Preoperative PET-CT imaging with increased metabolic activity in the area of the aortobifemoral Dacron® bypass as well as the enhancement surrounding the femoral chronic wound infection.
Bacteriophage Treatment
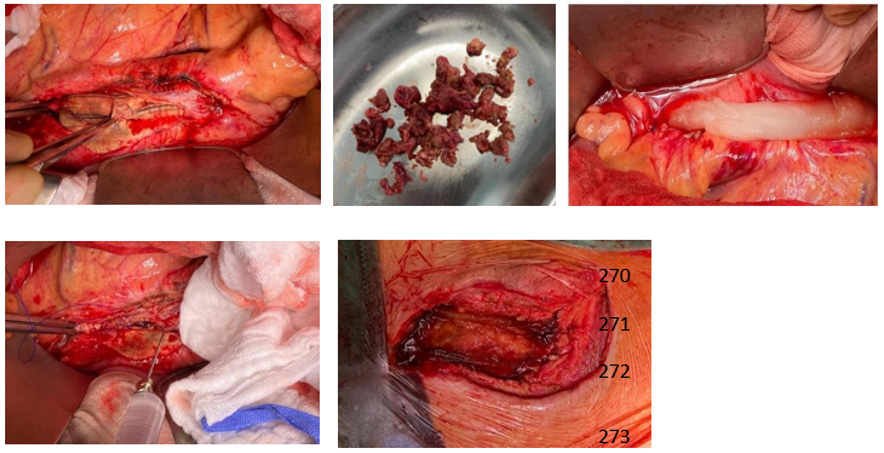
As a curative therapeutic strategy an intra and extra abdominal application of SniPha 360 (Phage24.com, Austria) was executed. SniPha 360 is a commercially available bacteriophage cocktail of lytic bacteriophages against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Streptococcus pyogenes, Proteus vulgaris and Proteus mirabilis. After outlining the potential risks but also benefits of the experimental procedure, the patient consented to the therapy. When performing the relaparotomy, cloudy fluid appeared within the abdomen. After an initial lavage, the retroperitoneum was opened, and the proximal aorta was prepared for clamping. The aorto- bifemoral Dacron® prosthesis presented a shell of a biofilm and was embedded in putrid fluid. The infected aortic prosthesis was extirpated, and the aorta was then sutured over. The prosthesis was retrieved femorally after mobilization of the legs of the prosthesis. The bacteriophage suspension was instilled on Tabotamb-Snow®, which was placed retroperitoneally around the infection.
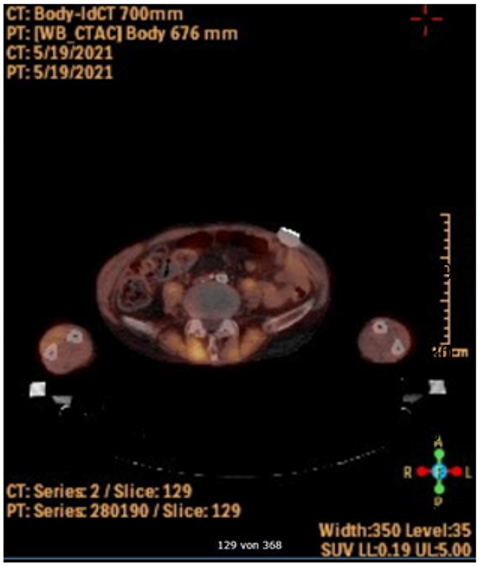
The retroperitoneum and abdomen were primarily closed without further drainage. After removing the femoral anastomoses, the wound conditions were debrided, mobilized and lavaged with a sharp spoon. A bacteriophage-soaked fleece was then placed bilaterally on the femoral side by the same principle, and the wounds were closed again without further drainage (Figure 2). The operation time was 52 minutes, without significant blood loss. Subsequently the patient could be taken to the intensive care unit and extubated without the need for catecholamines. After 10 days of hospitalization, the patient could be discharged with subjective well-being, irritation-free wound conditions and normal findings for inflammatory values in the blood. PET-CT imaging at three months post intervention did not show signs of infection enclosing the aorta or both femoral regions (Figure 3).
Figure 2: Intraoperative images
Intraoperative pictures showing infected aorto-bifemoral Dacron® bypass. Bacteriophage suspension application on a Tabotamb-Snow®, which was placed retroperitoneally.
Figure 3: Postoperative PET-CT
Postoperative PET-CT imaging showing no increased metabolic activity surrounding the bypass.
Discussion
This case demonstrates a successful treatment of a chronically infected occluded aorto-bifemoral Dacron® bypass by a local bacteriophage application. It is assumed that around 50-65% of prosthesis infections are a result of bacterial contamination during surgery [7-10]. A general distinction is made between early (up to 30 days postoperatively) and late infections, although the classification is arbitrary [7,10,11]. Early prosthesis infections are often assumed to be a consequence of intraoperative contamination and late infections to be a result of hematogenous bacterial spread, but profound evidence for this is limited. Late infections are usually caused by insufficient tissue integration of the prosthesis into the graft bed. Common pathogenic agents are staphylococci, enterobacteria and corynebacteria [7,10]. Bacteriophages (or simply ‘phages’; Greek: “bacteria eater”) are viruses that selectively infect bacterial cells and were first described in 1917 by the Canadian Félix Hubert d’Hérelle [12].
Currently Bacteriophages are known as a potent anti-bacterial treatment due to their lytic activity [13]. They are considerably stable when exposed to the inflammatory environment and contribute significantly to the regulation of global bacterial mass. A bacteriophage can only multiply where its host is. They are highly specific and therefore predominantly affect strains within one bacterial species, rarely crossing species boundaries [14]. In the first (lytic) cycle of viral reproduction, phages kill their corresponding bacteria through lysis: once infected, the bacterium host cell then starts the process of reproduction, the destruction of the bacterium, and the release of new phage particles; this process is controlled by enzymes and an interaction of bacterial and phage genes. In the second (lysogenic) cycle, the bacteriophage nucleic acid is integrated into the host bacterium’s genome or forms a circular replicon in the bacterial cytoplasm. Compared to other antibacterial therapeutic strategies like local Rifampine treatment [6], no cytotoxic effects on vascular cells could be found for bacteriophages [15].
In addition, they are effective on multi-drug resistant bacteria as well as biofilm-organized bacteria. Recently, in a case series of eight patients with infections of vascular grafts, surgical wounds or implanted medical devices further demonstrated the feasibility of using different bacteriophages with lytic activity for successful treatment of bacterial infections [16]. Although bacteriophages were used for successful treatment of infections of vascular implants, bacteriophage treatment is still not common and not an officially recommended option for infections in the westernized hemisphere [17]. The retro- and intraabdominal application of phages directly to the infection site ensured a maximum concentration, contact time and invasion of the bacteriophages into the infected peri graft tissue. We were able to perform a short operation time, a definite treatment in respect to complete skin/wound closure and the forego of any drainages. No bacteriophage related clinical adverse events had been detected in our case. A three-month follow-up PETCT scan revealed no signs of infections. It could be assumed that the bacteriophage treatment was successful.
In order to treat the local inflammation in the abdominal and femoral areas in the long term intra- and postoperatively, we perceived the use of bacteriophages as an alternative therapy option in antibacterial local therapy. However, there is an ongoing follow-up for the patient to assure a lasting treatment success. In summary, this case report demonstrates that bacteriophage treatment could be a curative treatment option for patients with bacterial graft- and peri graft infections that are not suitable for extensive surgical approaches.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.