Creation of Innovated Macrocyclic Sulfazan-Formazan Compounds and Linear Sulfazan-Formazan for the first Time Globally with their Assay as Antifungal
Introduction
Sulfazan compounds were invented for the first time globally by researcher Prof. Dr. Nagham Aljamali in 2019 was named as sulfazan compounds [1-3]. The researcher also developed and devised the basics of their preparation methods, the conditions of each method, their interactions, the reaction medium, and their naming. Then she developed them into cyclic sulfazan and cyclic formazan compounds [4-7] that were also prepared for the first time in April 2021 in this current study and also developed and established methods for their preparation and reaction conditions, and the current research included the preparation of innovative types of cyclic sulfazan compounds, linear sulfazan, cyclic formazan and linear formazan.
Sulfazan Compounds
Are organic sulfur compounds that were prepared in researches [1-3] as first time by Dr. Nagham Aljamali in 2019 from several reactions that include the reaction of the coupling of basic mercapto and thiol compounds with azo compounds using one of the conditions (pyridine, piperidine, triethylamine,...) [7-9] and its chemical structure is (Ar-N=N-S-R).
Formazan Compounds
Are a class of organic compounds of importance in organic chemistry because it contains two highly effective groups (-N=N=C-N-) or (-N=N-C-N-NH-) in several fields of chemistry [8-14], especially in coordination chemistry [15,16], as a ligand because they contain free electrons and donor atoms to coordinate with ions to form complexes [17-19] and types of them as anticancer [20-22].
Cyclic Formazan and Cyclic Sulfazan
These compounds invented by the researcher Dr. Nagham Aljamali in April 2021 for the first time [4,5]. Initial foundations and methods for preparing these compounds were established, and to determine the conditions [4-8] of their interaction and the auxiliary factors used to prepare them. They were considered among the organic compounds of importance in organic chemistry because they contain two highly effective groups in several fields of chemistry, especially in coordination chemistry, as a ligands because they contain doublets. Free electrons and donor atoms to coordinate with ions to form complexes [23-28]. Formazan also enter many anti-bacterial [29-33] and anti-fungal compounds [34- 37] and types of cancer [38,39], especially breast and laryngeal cancers, as anti-bacterial and anti-fungal, and other studies [40,41].
Instruments and Experimental Part
All melting points were uncorrected and dignified on an electrothermal apparatus (Switzerland) in an open capillary tube. FT.IR spectra were detailed on Fourier transform infrared spectrometer (FT-IR) in( FT-IR- 3600) infrared spectrometer via employing KBr Pellet technique., 1H.NMR spectra were recorded in DMSO-d6 as solvent using (TMS) as internal standard and chemical shifts are expressed as (δppm)., also Mass– Spectra for some of them other studies like evaluation against types of fungi).
Procedures
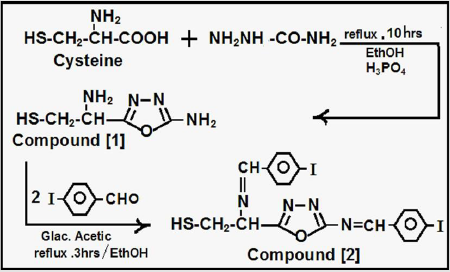
Preparation of Compounds{1, 2}
Cysteine (0.01 mole) was dissolved in (30ml) absolute ethanol with semicarbazide(0.01 mole) with refluxing for (10hrs) in presence of phosphoric acid as closing agent, according to procedures [6-9], the product filtered ,dried ,recrystallized to yield Oxadiazole amine Derivative-Compound [1], which reacted (0.01 mole) in (50 ml) absolute ethanol with p-iodobenzaldehyde (0.02 mole) with refluxing for (3hrs) in presence of (3 drops of glacial acetic acid), according to procedure [6- 9], the product filtered ,dried ,recrystallized to yield Aldamine -Compound [2] (Figure 1).
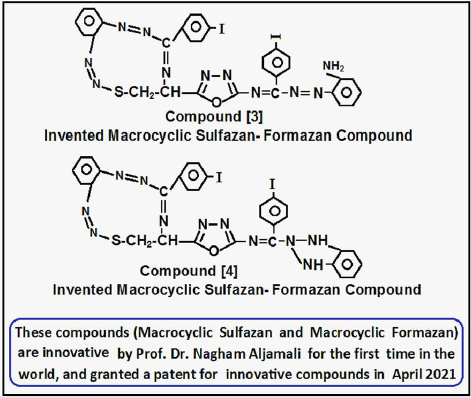
Creation of Inventive Macrocyclic Sulfazan-Formazan Compound{3}
Aldamine compound [2]was (0.01 mole) dissolved in basic solution from (Piprydine) then reacted with (0.02 mole) of diazo salt of o- phenyl diamine via many steps in basic solution to formation invented Macrocyclic Sulfazan-Formazan after (48hrs), the product filtered ,dried ,washed by distilled water, recrystallized to yield Invented Macrocyclic Sulfazan- Formazan [3] by following literatures [1-5].
Creation of Inventive Macrocyclic Sulfazan-Formazan Compound{4}
Macrocyclic Sulfazan-Formazan Compound{3} refluxed (0.01 mole) for (4hrs) in (40ml) absolute ethanol in presence of cupper acetate (0.92gm) in closing step of amine group in ortho- position from azo- group to formation Triazole derivative according to procedures [6,7] , the product filtered ,dried ,recrystallized to yield Invented Macrocyclic Sulfazan-formazan of Triazole derivative [4], according to procedures [6-10] (Figure 2).
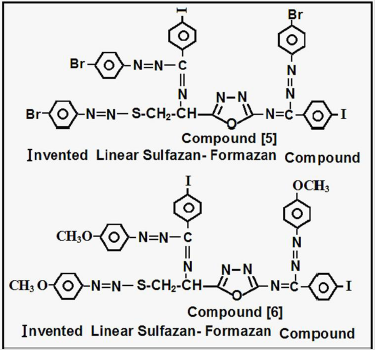
Creation of Inventive Linear Sulfazan- Formazan Compound{5}
Aldamine Compound [2] about (0.01 mole) was reacted basic solution (Pyridine) via many steps in coupling reaction with (0.03 mole) of p-bromophenyl diazonium salt in basic medium, after (10hrs), the product filtered ,dried ,washed by distilled water, recrystallized to yield Invented Sulfazan- Formazan [5], according to procedures [1-5].
Creation of Inventive Linear Sulfazan- Formazan Compound{6}
Aldamine Compound [2] about (0.01 mole) was reacted basic solution (Pipyridine) via many steps in coupling reaction with (0.03 mole) of p-methoxyphenyl diazonium salt in basic medium, after (10hrs), the product filtered ,dried ,washed by distilled water, recrystallized to yield Invented Sulfazan- Formazan [6], according to procedures [1-5] (Figure 3).
Results and Discussion
In recently study, various of Invented Macrocyclic Sulfazan- Formazan Compounds and linear Sulfazan- Formazan have been created in same procedure that followed and invented [1-5] by Dr. Nagham in year 2019 that got a patent to invention of Macrocyclic Sulfazan-Formazan compounds, then several studies were carried out to improve these innovative compounds by the using of spectral identification like : 1H.NMR spectra, FT.IR- Spectra, Mass- Spectra., other studies represented by (Melting points, evaluation against types of fungi)., all the results are revealed in Tables and figures.
Spectral Analysis
FT.IR- Spectral Evidence of Invented Macrocyclic Sulfazan- Formazan Compounds and Linear Sulfazan-Formazan Compounds : The first characterization of innovative compounds by shifting of frequencies of Aldamine group (CH=N) in starting compounds (Imine compounds) that were about at (1612) Cm-1 in starting compounds (imine compounds) were shifted to (1627) Cm-1 for (-C=N-) due to formation of Macrocyclic Formazan and band at (1310) Cm-1 due to (-S-N=N-) to Sulfazan group in Macrocyclic Sulfazan compound , also appearance of three bands due to partitions of azo group of Sulfazan and Formazan in Macrocycle (-N=N-) are (1423 ,1455, 1490) Cm-1 for (-N=N-S-)-Sulfazan and (-N=N-C-)-Formazan in compound {3}., While in compound [4] appeared band at (3298) Cm-1 due to (NH) in Triazole ring as a result of ring closure that resulted from closing of amine group in ortho- position with Azo group to formation Triazole ring, besides to appearance of bands at (1629) Cm-1 for (-C=N-) due to formation of Macrocyclic Formazan and band at (1304) Cm-1 due to (-S-N=N-) to Sulfazan group in Macrocyclic Sulfazan compound , also appearance of three bands due to partitions of azo group of Sulfazan and Formazan in Macrocycle (-N=N-) are (1432 ,1456, 1498) Cm-1 for (-N=N-S-)-Sulfazan and (-N=N-C-)-Formazan., and other compound like this., all frequencies explained according to reference [35].
1H.NMR- Spectral Evidence of Invented Macrocyclic Sulfazan-Formazan Compounds and Linear Sulfazan- Formazan Compounds
The second characterization of innovative compounds by disappearance of peak for imine group (CH=N) in starting compound (Aldamine compound) that were at δ (8.27) in Compound {2} (starting compound) due to formation of (N=C-N=N) for (Formazan and Sulfazan groups) in created compounds, also in compound [4] appeared peak at δ (5.35) due to (NH) in Triazole ring as a result of ring closure that resulted from closing of amine group in ortho- position with Azo group to formation Triazole ring, all peaks explained according to reference [35].
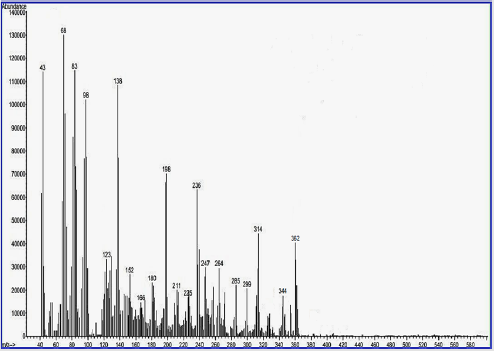
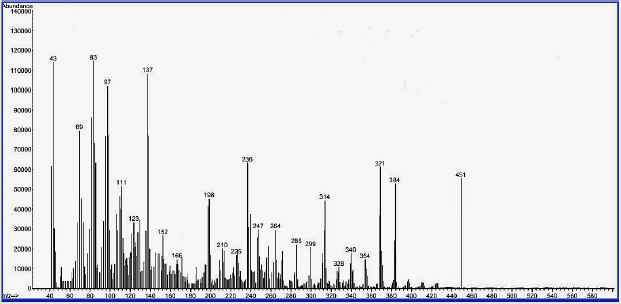
Mass– Spectral Evidence of Invented Macrocyclic Sulfazan-Formazan Compounds and Linear Sulfazan- Formazan Compounds
The third characterization of inventive compounds by partition of innovative cyclic compounds via appearance of fragments in spectra in Figures 4-6.
Other Characterization
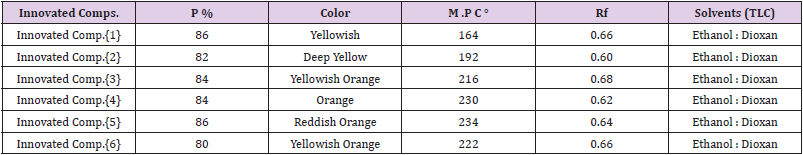
All Invented Macrocyclic Sulfazan- Formazan and Linear Sulfazan-Formazan were studied to collect all The chemical and physical properties, in Table 1.
Antifungal Assay [17,18] of Invented Sulfazan-Formazan Derivatives
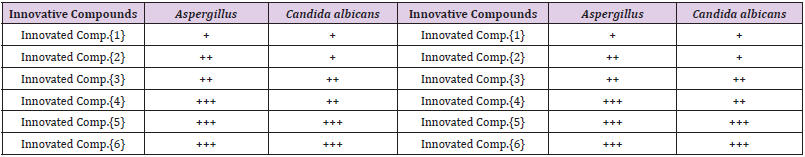
The assessment of Invented Macrocyclic Sulfazan-Formazan derivatives and Invented Linear Sulfazan-Formazan derivatives tested against kinds of fungi epitomized through (Aspergillus) with (Candida Albicans) for all the invented compounds at three concentrations were occupied range of three analyses that occupied for each concentration (20, 30, 50μgm) rendering to the method [17,18], Table 2. The results improved that the Invented Macrocyclic Sulfazan-Formazan compounds and Invented Linear Sulfazan- Formazan compounds have good results as inhibitor of fungi growth and the invented macrocyclic Sulfazan- Formazan compound [5] has more activity than other invented macrocyclic Sulfazan-formazan compound due to structure of compound [5] involved sufazan group (-N=N-S-) with formazan group (-N=CN= N-) more than other compounds besides to (Br) atoms , while compound [6] has less inhibition activity due to its structure ( -OCH3) methoxy group.
Table 2: Antifungal Assay of Invented Sulfazan-Formazan Compounds in Concentration (30μ.gm).
Note:
(+) : inhibition (4-7)mm
(++) : inhibition (8-13)mm
(+++) : inhibition (14-18)mm
Conclusion
All Invented Macrocyclic Sulfazan-Formazan derivatives and Invented Linear Sulfazan-Formazan compounds gave good evidences for their structures via various spectral techniques, also some of them studied against types of fungi that gave good data and good activity in inhibition of growth of Fungi.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.