Experimental Cerebral Ischemia Causes Disturbances in Mitochondrial Respiration of Neurons
Introduction
Energy exchange in the cell is associated with mitochondria, which play an important role in vital processes, participating not only in the formation of ATP, but also in the storage and transmission of hereditary information, apoptosis and plastic processes [1-3]. Mitochondria are very mobile and plastic organelles that constantly change their shape, have the ability to fusion and subsequent separation. The movement of mitochondria in the cytoplasm is associated with microtubules, which determines their orientation and distribution in the cell. In some cells, mitochondria form long mobile filaments or chains, while in others they are fixed near the places of consumption of ATP [4-6]. Neurons need a constant supply of ATP for their stability and maintaining the level of potassium ions K + inside the cell, and sodium and calcium ions outside. At rest, the brain consumes up to 20% of the oxygen received by the body. Under normal conditions, effective biological oxidation is the main source of energy-rich phosphate compounds required for the renewal of structures corresponding to the functional activity of cells [5-7]. Elucidation of the mechanisms of development of energy deficiency in ischemic damage is advisable for detailing the pathogenesis, the ratio of damage and compensation processes in this pathology.
Materials and Research Methods
The experiments were carried out on 88 male outbred white rats weighing 260 ± 20 g in compliance with the Directive of the European Parliament and of the Council No. 2010/63/EU of 22.09.2010 on the protection of animals used for scientific purposes. The studies used models of total (TCI) and subtotal (SCI) cerebral ischemia. Cerebral ischemia (CI) was modeled under conditions of intravenous thiopental anesthesia (40-50 mg/kg). Total cerebral ischemia was modeled by decapitation of animals. Subtotal cerebral ischemia was modeled by simultaneous ligation of both common carotid arteries (CCA). The sampling of material for the study of tissue respiration of mitochondria was carried out 1 hour and 24 hours after decapitation or ligation of the CCA. The control group consisted of sham-operated rats of the same sex and weight. To study mitochondrial respiration, the brain was removed in the cold (0-4°C), dried with filter paper, weighed and homogenized in an isolation medium containing 0.32 M sucrose, 10 mM Tris-HCl, 1 mM EDTA, pH 7.4 (in the ratio 1:10) using a Potter-Evelheim homogenizer with a Teflon pestle according to the modified method [8]. Mitochondria were isolated by differential centrifugation.
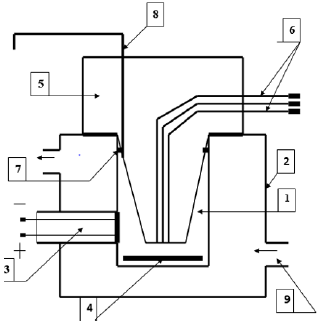
The nuclear fraction was separated by centrifugation at 600g for 10min (4°C). The resulting supernatant was centrifuged at 8500g for 10min (4°C), the mitochondrial pellet was washed twice in the isolation medium and resuspended to a protein concentration of 35-40mg/ml in the isolation medium and stored in a short tube on ice. Protein concentration was determined by the Lowry method. To study mitochondrial respiration, a concentrated suspension of mitochondria was introduced into a thermostated sealed polarographic cell with an incubation medium in an amount providing a final protein concentration in the cell of 1mg/ml. The incubation medium for recording mitochondrial respiration includes 0.17 M sucrose, 40 mM KCl, 10 mM Tris-HCl, 5 mM KH2PO4, 8 mM KHCO3, 0.1 mM EDTA, pH 7.4. The principle of operation of a 3.0 ml polarographic cell is based on the registration of oxygen uptake by mitochondria using a built-in Clarke electrode at a temperature of 25°C (Figure 1).
Figure 1: Polarographic cell for studying the respiratory activity of mitochondria
1. Cell;
2. Thermostatically controlled chamber;
3. Clarke electrode;
4. Mmagnetic stirrer;
5. Sealing plug;
6. Channels for dosed anaerobic administration of substrates and ADP;
7. A sealing ring;
8. Channel for removing air and excess liquid;
9. Fitting for connection to an ultrathermostat.
Registration of changes in oxygen tension (pO2) in the mitochondrial suspension was carried out using an electronic recorder KSP-4. The Clarke electrode was calibrated by successively blowing air (pO2 of air) and gaseous nitrogen (pO2 = 0 mmHg) through the cell. After recording the rate of basal (endogenous) respiration in the absence of a substrate (V1), respiration substrates (malate - 2 mM/glutamate - 5 mM or succinate - 5 mM) are alternately introduced into the mitochondrial suspension, and then ADP in an amount of 200 nmol/ml. The obtained polarograms are used to calculate the respiration rate of mitochondria in different metabolic states and the coefficients characterizing the conjugation of oxidation and phosphorylation processes. The following indicators of mitochondrial respiration were recorded: V1 - the rate of basal respiration, V2 - the rate of substrate-dependent respiration, V3- - the rate of respiration coupled with phosphorylation (after the addition of ADP), V4 - the rate of respiration after the completion of phosphorylation of the added ADP.
The indicators characterizing the conjugation of oxidation and phosphorylation processes in mitochondria were determined: the acceptor control coefficient (AK = V3/V2), the respiratory control coefficient (DC = V3/V4) and the phosphorylation coefficient - ADP/O. The use of solutions of substrates of the complex “malate/ glutamate” and succinate makes it possible to assess the degree of functional activity of the electron transfer chain (ETC) in mitochondria in general, in particular I and II of the ETC complex [9,10]. To prevent systematic measurement errors, brain samples from the compared control and experimental groups of animals were studied under the same conditions. As a result of research, quantitative continuous data were obtained. Since the experiment used small samples that had an abnormal distribution, the analysis was carried out by methods of nonparametric statistics using the licensed computer program Statistica 10.0 for Windows (StatSoft, Inc., USA). Data are presented as Me (LQ; UQ), where Me is the median, LQ is the value of the lower quartile; UQ is the upper quartile value. Differences between groups were considered significant at p<0.05 (Kruskell-Wallis test with Bonferoni’s correction).
Results
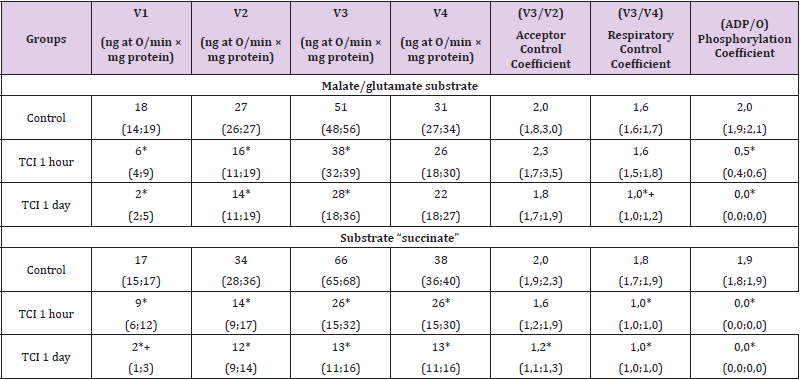
Compared with the control, at 1-hour TCI in the presence of the substrate “malate/glutamate”, which characterizes the state of the first (NADH-dehydrogenase) complex, the electron transport chain V1 decreased by 65 (58; 67)%, p<0.05, V2 - by 41 (38; 48)%, p<0.05, V3 - by 25 (22; 38)%, p<0.05, and the phosphorylation coefficient - by 78 (71; 84)%, p<0,05. The rest of the indicators (V4, the coefficient of acceptor control and the coefficient of respiratory control) did not change (p>0.05), (Table 1). In the presence of succinate substrate, which reflects the work of complex II (succinate dehydrogenase) of the electron transport chain, a decrease in the parameters of energy exchange was established: the rate of basal respiration V1 – by 44 (38; 52)%, p<0.05, the rate of substratedependent respiration V2 – by 60 (48; 64)%, p<0.05, respiration rate associated with V3 phosphorylation - by 59 (38; 65)%, p<0.05, respiration rate after completion of phosphorylation of added V4 ADP – by 32 (28; 46)%, p<0.05 [9].
Table 1: Respiration indices of the mitochondrial fraction of rat brain homogenates with total cerebral ischemia when using the substrates “malate/glutamate” and “succinate”, Me (LQ; UQ).
Note: p<0.05 compared with the control group, + – p<0.05 compared with 1-hour TCI, TCI - total cerebral ischemia.
The respiratory control coefficient (V3/V4) decreased by 45 (42; 48) % (p<0.05), the phosphorylation coefficient (ADP/O) at 1-hour CTI was zero. The acceptor control coefficient (V3/V2) did not change (p>0.05). The decrease in the rate of basal respiration V1 was more pronounced when using the substrate succinate (by 21%, p<0.05), which indicates a greater damage to the II complex (succinate dehydrogenase) of the electron transport chain (ETC) during TCI. There were no differences between other indicators (p>0.05). Under conditions of 1-day TCI in the presence of the substrate “malate/glutamate” at 1-day TCI, V1 decreased by 90 (82; 96) %, p<0.05, V2 – by 46 (42; 55) %, p<0,05, and V3 – by 45 (39; 56) % (p<0.05), the respiratory control coefficient – by 35 (31; 42) % (p<0.05). Indicators V4 and the coefficient of acceptor control did not change (p>0.05). The phosphorylation coefficient (ADP/O) when using both succinate and malate/glutamate with 1-day TCI, as with 1-hour TCI, was equal to zero. Compared to 1-hour TCI, the respiratory control coefficient with 1-day TCI was 41 (35; 47) % less (p<0.05).
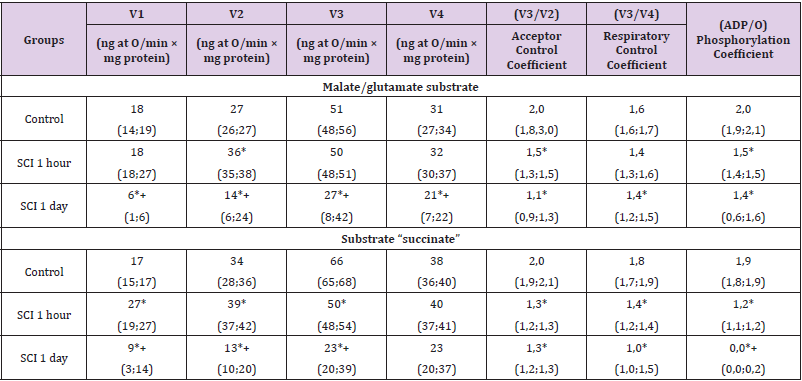
There were no differences in other indicators (V2, V3¬, V4, acceptor coefficient and phosphorylation coefficient) (p>0.05), (Table 2). In the presence of succinate, there was a more significant decrease in V1 than with 1-hour TCI - by 90 (84; 95) %, p<0.05, V2 - by 65 (54; 70) %, p<0.05, V3 - by 78 (54; 87) %, p<0.05, V4 - by 67 (59; 81) %, p<0.05. The respiratory control coefficient decreased by 45 (41; 49) %, p<0.05. Compared with 1-hour TCI, the V1 index was 80 (75; 86) % less (p<0.05) Changes in mitochondrial respiration in relation to the control level when using both substrates were equivalent (p>0.05). In the SCI group lasting 1 hour, compared with the control group, in the presence of malate/ glutamate, V2 increased by 24 (18; 27) %, p<0.05, and the acceptor control coefficient and phosphorylation coefficient decreased by 25 (17; 29) %, p<0.05. Other indicators (V1, V3, V4, respiratory control coefficient) did not change (p>0.05). In the presence of the substrate “malate/glutamate” at 1-hour SCI, the mitochondrial respiration indices V1, V2, V3 and V4 were 89 (82; 93) % higher than at 1-hour TCI, p<0.05, 58 (55; 63) %, p<0.05, 24 (21; 29) %, p<0.05 and 32 (27; 37) %, p<0.05, respectively [10-19].
Table 2: Respiration indices of the mitochondrial fraction of rat brain homogenates in subtotal cerebral ischemia when using the substrates “malate/glutamate” and “succinate”, Me (LQ; UQ).
Note: p<0.05 - in relation to the control level, + – p<0.05 compared to 1-hour SCI, SCI - subtotal cerebral ischemia
The respiratory control coefficient did not change (p>0.05), while the acceptor control coefficient was 34 (24; 43) % less, p<0.05, and the phosphorylation coefficient was 66 (58; 73) % more, p<0.05. The decrease in the phosphorylation coefficient with SCI was less significant by 53% (p<0.05). In the presence of succinate substrate, an increase in the rate of basal respiration V1 was noted - by 38 (34; 42) %, p<0.05, the rate of substratedependent respiration V2 - by 13 (9; 18) %, p<0.05, rate respiration associated with V3 phosphorylation - by 26 (21; 32) %, p<0.05. These changes indicate a significant decoupling of oxidation and phosphorylation. The respiration rate after the completion of phosphorylation of the added ADP (V4) did not change (p>0.05). At the same time, the acceptor control coefficient, the respiratory control coefficient and the phosphorylation coefficient decreased by 35 (31; 39) %, p<0.05, 20 (18; 28) %, p<0.05 and by 36 (30; 41) %, p<0.05, respectively, which indicates a decrease in energy production.
Compared with 1-hour TCI, with 1-hour SCI in the presence of the substrate “succinate” the rates V1, V2¬, V3 and V4 were 67 (62; 71) % higher, p<0.05, 64 (58; 68) %, p<0.05, 46 (39; 52) %, p<0.05 and 35 (31; 41) %, p<0.05, respectively. The respiratory control coefficient increased by 30 (24; 36) %, p<0.05. The phosphorylation coefficient at 1-hour TCI was zero. When using succinate, the decrease in the respiratory control coefficient was less pronounced with SCI (by 10%, p<0.05). When using succinate, when using malate/glutamate, in relation to the control level, the phosphorylation coefficient was lower by 11% (p<0.05). Other indicators (V1, V3¬, V4, respiratory control coefficient, acceptor control coefficient) did not differ (p>0.05). An increase in V1 and V2 and a decrease in the phosphorylation coefficient (ADP/O) indicates proton transfer bypassing the ATP synthase complex. Enzymes of the mitochondrial matrix and cytochrome in this model of CI do not yet have pronounced damage, as evidenced by the high rates of V1 and V2, however, a decrease in the coefficients of acceptor control, respiratory control and phosphorylation indicates the separation of oxidation and phosphorylation processes and a decrease in the production of ATP during SCI.
More pronounced disturbances with the use of succinate than with the use of malate/glutamate indicate a greater damage to the succinate dehydrogenase complex of ETC, as in TCI. In the presence of the substrate “malate/glutamate” at 1-day SCI, compared with 1-day TCI, V1 decreased by 66 (60; 71)%, p<0.05, V2 - by 45 (41; 50)%, p<0.05, V3 - by 47 (39; 52)%, p<0.05, V4 - by 34 (27; 39)%, p<0.05, which is more significant than with 1-hour SCI by 87 (72; 94) %, p<0.05, 61 (58; 73)%, p<0.05 and by 46 (41; 52)%, p<0.05, respectively, except for the value of the V4 indicator, which did not change, p>0.05. The coefficients of acceptor control, respiratory control and phosphorylation decreased by 42 (37; 51) %, p<0.0; by 12 (9; 18) %, p<0.05 and by 25 (21; 32) %, p<0.05, respectively. In the presence of the “malate/glutamate” substrate, the basal respiration rate V1 was 67% higher (p<0.05), the acceptor control coefficient was 39% lower (p<0.05), and the phosphorylation coefficient at 1-day TCI was zero. Under the conditions of daily SCI in the presence of succinate substrate, a decrease in V1 was noted - by 47 (39; 51)%, p<0.05, V2 - by 62 (54; 66)%, p<0.05, V3 - by 64 (59 ; 68)%, p<0.05, which is more pronounced than with 1-hour SCI by 67 (62; 72)%, p<0.05; by 66 (63; 74)%, p<0.05 and by 55 (49; 59)%, p<0.05, respectively.
The acceptor control coefficient and the respiratory control coefficient decreased by 35 (29; 41) %, p<0.05 and by 44 (38; 49) %, p<0.05, respectively. The phosphorylation coefficient with 1-day SCI, as well as with 1-day TCI, was equal to zero. Compared with TCI lasting 1 day, with 1-day SCI in the presence of succinate substrate, the V1 rate was 78% higher (p<0.05), and the V3 and V4 rates were higher by 43% (p<0.05). while other parameters did not change (p>0.05). The use of a mixture of malate with glutamate as a substrate for 1-day SCI showed similar changes in mitochondrial respiration parameters as with succinate, with the exception of a higher phosphorylation coefficient - 1.4 (0.6; 1.6), p<0,05. The decrease in the V1, V2, and V3 indices with 1-day SCI is a consequence of a decrease in the oxygen content for mitochondrial respiration.
The suppression of energy processes was more pronounced than with 1-hour SCI, which reflects an extremely low phosphorylation coefficient. Changes in V1, V2 and V3 indicators at 1-hour SCI and 1-hour TCI were multidirectional. Their increase in SCI is associated with the uncoupling of oxidation and phosphorylation, while a decrease in TCI is associated with a lack of substrates for mitochondrial respiration. The decrease in the rate of basal respiration V1 with 1-day SCI was less pronounced than with TCI: in the presence of succinate - by 43% (p<0.05), and in the presence of malate/glutamate - by 24% (p<0.05).
Conclusion
During cerebral ischemia, damage to the inner mitochondrial membrane occurs due to the activation of free radical oxidation processes [5]. Damage to the inner mitochondrial membrane, in turn, leads to an increase in its permeability and a decrease in the level of the proton gradient due to the transition of protons along the concentration gradient through the formed nonspecific pores into the mitochondrial matrix [13,19,18]. As a result, the efficiency of ATP synthesis decreases, and to maintain the intermembrane potential under these conditions, more substrates and oxygen are required [14-18]. Thus, the most pronounced decrease in the respiration indices of the mitochondrial fraction of brain homogenates occurs in total cerebral ischemia due to the complete cessation of the blood supply to the brain neurons.
With this method of modeling cerebral ischemia, the appearance of hyperchromic shriveled neurons with pericellular edema is characteristic. In their cytoplasm, the destruction of organelles occurs, the disintegration of neurofibrils and neuropil, which indicates their low functional activity. Simultaneous subtotal ischemia also leads to severe irreversible damage to neurons: at the morphological level, this is manifested by a significant increase in the number of hyperchromic shriveled neurons. Their predominance in the population of neurons in the SCI group corresponds to the inhibition of respiration of the mitochondrial fraction of brain homogenates.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.