Pfizer/Biontech Post Vaccine Acute St-Elevation Myocardial Infarction: A Case
Introduction
Corona-type viruses have been known for years. In the 2019/2020 period, a new type of SARS-Corona virus-2 (SARS-CoV- 2=COVID-19) started to spread all over the world. Several vaccines have been approved against COVID-19. Each of these sought to respond to the pandemic by providing individual protection against COVID-19. One of these vaccines is the messenger ribonucleic acid (mRNA) COVID-19 (Pfizer/BioNTech) vaccine that is based on new gene-based technology. It was recommended that the vaccine be administered in two doses, with a 21-day interval between them. Based on current knowledge, COVID-19 mRNA vaccines have a high efficacy rate of approximately 95%. Current research data indicate that the odds of contracting COVID-19 infection are approximately 95% lower in fully vaccinated persons than in unvaccinated persons. Its effectiveness in preventing severe COVID-19 disease (for example, hospitalization) is about 85%. It is not yet known how long the protection of this vaccine will last. The Pfizer/BioNTech vaccine is approved for people 12 years of age and older. However, some unexpected side effects may occur after vaccinations for COVID-19 infection, the future of which is still unknown. After one of the mRNA vaccines, local and general reactions can be seen as a result of the interaction of the vaccine with the body. These reactions are generally expected to disappear within 2 days after vaccination [1].
Although rare cases of myocarditis and pericarditis have been reported among cardiac side effects after the mRNA covid-19 vaccine, no significant heart attack was observed in vaccinated patients. It is thought that other medical conditions that develop during or after vaccination may not always be related to the vaccine, since large-scale vaccination is performed [2]. Diagnosing postvaccine Acute Myocardial İnfarction (AMI) can be difficult. Because muscle pain at the injection site may cause ischemic symptoms to be overlooked and delayed admission. As far as we can search the literature, we know of two cases of acute myocardial infarction in the United States that started less than 24 hours after the first dose of the COVID-19 vaccine [3]. In our case, we aimed to emphasize acute myocardial infarction and other possible side effects that developed within hours after the second dose of Pfizer/BioNTech COVID-19 vaccine, in the light of the literature.
Case Report
A 39-year-old male patient said he had shoulder, arm, and chest pain lasting more than three days after receiving the first dose of the Pfizer/BioNTech COVID-19 vaccine. There was a relief after taking paracetamol and nonsteroidal anti-inflammatory tablets for his pain. He attributed all these pains to possible post-vaccine reasons. After 21 days, the patient applied to the emergency service with complaints of discomfort and tightness in the chest, which started 1.5-2 hours after the second dose of vaccine, continued for more than 10 minutes. Also, the patient could not describe the nature of the pain exactly. He stated that the pain was not like in the first dose, but was suppressive, restless, starting from the scaphoid and spreading to the jaw and neck. The patient had both vaccines done on the left arm. The patient did not have the fever or respiratory distress at the time of admission and did not report any drug-vaccine allergy. There was no cardiac history in his own and first-degree relatives. In his examination; general condition was good. Vital signs are blood pressure; arterial 130/70mmHg, temperature: 36.5 °C, oxygen saturation parameter (SpO2): 99%, heart rate: 70/minute. When the heart was listened to, there was no additional sound and no murmur. Peripheral pulses were palpable. Other system examinations were normal. Electrocardiography (ECG), complete blood count, biochemistry, and cardiac biomarkers were requested from the patient. Polymerase Chain Reaction (PCR) was requested for COVID-19 in the nasopharyngeal aspirate in the emergency department, the test result was negative.
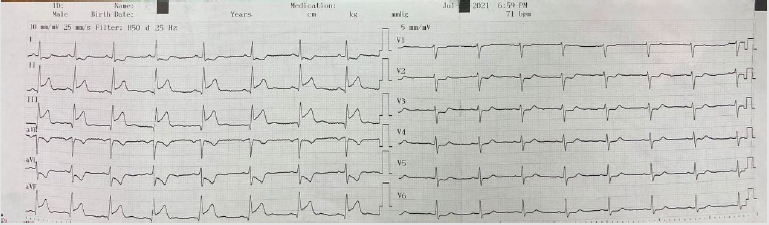
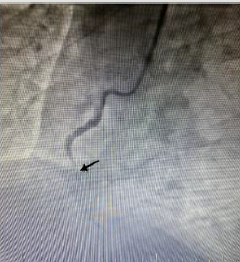
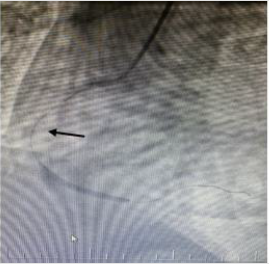
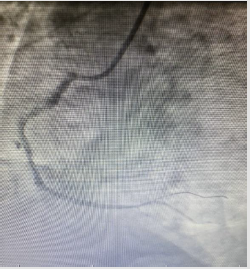
In the ECG taken in the fifth minute of the patient’s admission to the emergency department; ST-segment elevation in inferior leads (II, III, and aVF) and reciprocal ST-segment depression in I and aVL were detected (Figure 1). The patient’s cardiac troponin T value at admission; 8.83pg/dl (range: 0-14pg/ml), 5097pg/dl at the 6th hour and 2689pg/dl at the 24th hour. ECG monitoring, 300 mg acetylsalicylic acid, 300 mg clopidogrel orally, 5000 IU heparin intravenously were administered to the patient with the diagnosis of acute inferior myocardial infarction. Emergency coronary angiography showed globular thrombus and occluded proximal segment of the Right Coronary Artery (RCA) with TIMI 0 flow (Figure 2). RCA emergency Percutaneous Coronary İntervention (PCI) was performed. Dilated with balloon and stent placed. After coronary angiography, a good outcome was detected with the TIMI III flow (Figures 3 & 4). The patient did not have chest pain after percutaneous coronary intervention. On the fifth day of hospitalization, the patient had minimal hypokinesia of the right inferior wall and was discharged with a cured left ventricular ejection fraction of 60-65%.
Figure 1: ST elevation in lead II, III and AVF (blue arrow) and ST depression in I and AVL (red arrow).
Discussion
Although vaccines dedicated to COVID-19 disease have been approved as safe, both the disease and the vaccine are relatively new [4-6]. This requires that even very rare events be widely shared and discussed with the medical community. We report a case of AMI occurring within two hours of the second dose of the COVID-19 vaccine. The angiographic features of the case suggested acute thrombotic events as the underlying mechanism. Although it is not possible to establish a causal relationship between vaccination and cardiac events, it is important to remember that the mechanisms of thrombotic events in COVID-19 are still not fully understood [7]. Vaccines are considered the most effective drugs in public health. Side effects may also occur due to some reactions after vaccination. It is often difficult to say whether the reactions are caused by the vaccine itself or by other factors causing AMI. Excipients used in vaccines; it is used to improve stability, increase solubility and improve absorption. However, these substances contribute significantly to the development of IgE-mediated anaphylactic reactions during vaccination [8].
Kounis syndrome is an allergic reaction to various substances, including excipients, resulting in acute coronary syndrome [9]. This could be a possible explanation for the AMI clinic post- COVID-19 vaccine. It may also occur through prothrombotic immune thrombocytopenia, which is similar to heparin-induced thrombocytopenia, leads to vaccine-induced thrombotic events [10]. Although there is limited data on the risk of thrombotic events with vaccines currently in use in the United States, there have been recent reports of thrombotic events after COVID-19 vaccines in Europe. No acute myocardial infarction was reported in the original study of vaccines used in Covid-19. They also did not present a significant difference in thrombotic events between the vaccinated and placebo groups. However, cases have started to be presented after these vaccines, rarely. Boivin et al. reported a 96-year-old female case who had AMI one hour after the Moderna COVID-19 vaccine, and one case of AMI in a 63-year-old healthy man two days after the AstraZeneca COVID-19 vaccine [11]. Maadarani AstraZeneca presented a case of AMI after the COVID-19 vaccine [12]. Tajstra on the other hand, reported an 86-year-old male case of AMI after Pfizer/BioNTech Covid-19 vaccine [13]. Finally, Jonathan presented two cases of AMI, one male, and one female, after the Moderna vaccine [3]. Our case can be seen as the second case in the literature after Pfizer/BioNTech Covid-19 vaccine. The Vaccine Adverse Event Reporting System (VAERS) [14], the National Vaccine Safety passive monitoring system, reported that side effects occurred after receiving the second dose of 76% of mRNA vaccines made so far. According to the Centers for Disease Control and Prevention (CDC) case definitions, The median interval from vaccination to symptom onset was 2 days (range = 0-40 days). Some of the symptoms were seen within 7 days of vaccination in 92% of patients. Acute clinical courses were generally mild [15]. About 78% of Suspected Adverse Events (AEs) were reported in individuals younger than 60 years of age. In clinical trials of mRNA vaccines, it has been noted that people younger than 60 years tend to experience more reactogenic AEs than those aged 60 and over. In general, younger individuals have more active immune responses and may experience more AEs to vaccines. This is part of the body’s natural response to build immunity to COVID-19 infection. Approximately 66% of AEs have been reported in women.
Of the 7.5 million doses of Pfizer-BioNTech and Moderna mRNA vaccine administered in total, 0.12% were reported as suspicious and 0.005% as severe AEs. The most commonly reported serious side effects are anaphylaxis and other serious allergic reactions. Also asthma exacerbation, difficulty breathing, fast heart rate, increase or decrease in blood pressure, chest discomfort and pain, pericarditis or myocarditis, syncope, numbness of limb, weakness or pain, vision changes, increase in liver enzymes, thyroid gland dysfunction, muscle injury, joint pain, seizures, tinnitus, infections, and blood clots. Most individuals who develop severe AE are reported to have recovered. A higher incidence of heart attacks and strokes was not observed in locally vaccinated persons. It’s also important to note that heart attacks and strokes can occur naturally, regardless of whether people are vaccinated or not. It is argued that the benefits of COVID-19 vaccines continue to outweigh the known risks in the pandemic [2].
Conclusion
As the COVID-19 vaccine campaign moves into a new phase worldwide and vaccines become available to the general public, there will be more data on safety and potential side effects. Further data on nationwide myocardial infarction or other thrombotic events before and after vaccination initiation should be carefully considered and interpreted before commenting on any possible causal relationship.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.