Cardioprotection with Inhaled Anesthetics
Introduction
Cardiovascular diseases are a group of disorders of the heart and blood vessels. According to PAHO, each year more people die from cardiovascular diseases than from any other cause. By 2020, deaths from these diseases will increase by 15 to 20%, and in 2030, approximately 23.6 million people will die from them, which makes it persist as the leading cause of death worldwide. These disorders consist of acute episodes that affect vascular perfusion through obstructions that alter the normal flow of blood to organs such as the heart and brain; caused by multiple risk factors that induce the formation of atheromatous plaques on the walls of blood vessels. Among the factors involved are smoking, obesity, sedentary lifestyle, diabetes and hyperlipidemia. [1] Volatile anesthetics are gases that induce general anesthesia, with rapid onset and recovery. In addition, its effect is less in the peripheral organs. In 1986, Reimer studied the decrease in ATP at the origin of myocardial damage in dogs, exposing them to a short period of ischemia, which showed that the more exposure there was, the less aggravation of the infarct zone. Then Murry continued the studies based on the fact that myocardial cells adapt as they present a repetitive noxious stimulus, by reducing energy consumption. Murry determined the adaptation mechanisms that participate in this, such as the reduction in the consumption of ATP and the limitation of the accumulation of catabolites during ischemia, which is summarized as a protection against ischemic damage. From all this arose pre and post ischemic conditioning, which resemble the behavior of halogenated gases and their cardioprotective effect, acting on channels sensitive to ATP. Many studies have commented on the effect of these agents on the myocardium, showing the beneficial action of the pharmacological mechanism when used before and after ischemia in patients with ischemic heart disease [2,3].
Materials and Methods
A narrative review was carried out, in which databases such as Scielo, PubMed, Sciencedirect, academic google were used. A search was carried out for articles in journals indexed in English and Spanish languages from 2012 to 2021. The keywords used were according to the MeSH terms. They were used: cardioprotection, anesthetics, halogenated, myocardial ischemia, adenosine. In this review, 21 original and review publications related to the subject under study were identified, of which 14 met the inclusion criteria used. Among the inclusion criteria are that they were full-text articles, that at the time of the search they allowed the reading of the abstract, that they were related to the subject studied and that they were within the established years. Exclusion criteria: That their publication date was less than 2012 and that they allowed the full text to be read.
Results
Ischemic Preconditioning
Ischemic preconditioning was studied in 1986 by Murry, et al. Ischemia occurs when there is a decrease or interruption in the supply of oxygen to a tissue, which can generate tissue necrosis, when compensatory mechanisms such as the use of energy reserves are exhausted, finally leading to irreversible damage and later to death. However, in preconditioning, tissue involvement is transitory, there is an injury to the tissue in order to prepare it or protect it from further damage, since it helps the cardiac cells to contract with less effort and less energy consumption. Thus, a short period of ischemia is preferable to a prolonged one, for which volatile anesthetics confer cardioprotection even though the mechanisms involved in it are not yet clear. In other words, in preconditioning there is an exposure to a harmful stimulus which will generate an increase in cellular resistance to subsequent exposure. This occurs in two phases: the initial phase, which is immediate, confers a short protection of 1 to 2 hours, and a late phase lasting approximately 24 hours. In the late phase, cellular memory is formed, it is believed that it is thanks to the activation of proteins that have a cytoprotective effect. In ischemic postconditioning, short ischemic episodes would be applied in the initial phase of reperfusion, thus reducing the extent of cellular lesions that generally occur in that period [2,4].
Reperfusion Injury
The purpose of reperfusion is to reduce ischemia; however, this can trigger a series of myocardial injuries, predisposing to a high risk of suffering myocardial infarction within the first 24 hours after surgery. These changes consist of arrhythmias, accelerated necrosis, and lethal damage, as reported in animal studies. All this due to mechanisms of endothelial dysfunction, accumulation of ions and free radicals, which generate a pro-inflammatory response. Volatile anesthetics confer cardioprotective effects against Ischemia-Reperfusion injury, when administered shortly before ischemia; This effect arises from 12 hours after the administration of these drugs and can last up to 72 hours. Preconditioning exerts delayed cardioprotective effects against myocardial injury in rats by activating AMPK (AMP-activated protein kinase) and restoring altered autophagic flow [5,6].
Cardiac Ischemia
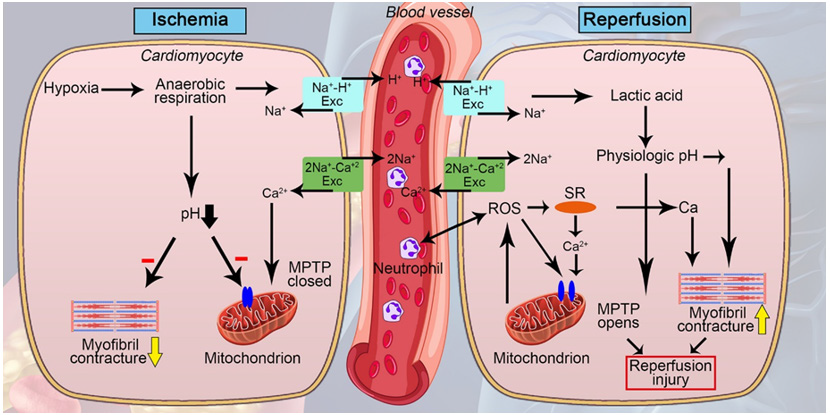
When cardiac ischemia occurs, myocytes suffer from a lack of energy, which creates an anaerobic state with a subsequent increase in lactic acid and a decrease in pH. The reserved adenosine triphosphate is consumed, and reactive oxygen species begin to be produced, since the mitochondria that normally produce ATP within cardiac cells, during ischemia have a high oxygen requirement. In addition, this loss of ATP generates ionic disorders since the Na / k pump is affected, which culminates in a decrease in intracellular sodium and an increase in calcium, deteriorating the cell membrane and causing apoptosis. Regarding reperfusion, it also causes calcium overload, inadequate ATP synthesis, low nitric oxide production, and oxidative stress. In this reperfusion process, the increase in ATP is harmful, since it generates hypercontracture of the cardiomyocytes, breaking the membrane and necrotizing the bands [7].
Anesthetic Preconditioning
When volatile anesthetics are administered before or after periods of ischemia, they exert protective effects on cells through various mechanisms. These mechanisms include modulation of G protein-coupled receptors, intracellular signaling pathways, gene expression, potassium channels, and mitochondrial function. In the preconditioning mechanism, mediators are activated that confer cardioprotection during the period of ischemia and reperfusion; volatile anesthetics are lipophilic and can diffuse into cell membranes, acting as preconditioning inducing agents, leading to the activation of protein C kinase, the opening of potassium channels, and differential genetic regulation. Currently it is believed that halogenates act directly, since they not only decrease contractility and oxygen consumption, but also occur by the activation of intracellular pathways that prevent myocardial necrosis. [3,7] Halogenated anesthetics protect against ischemiareperfusion injuries, they provide protection against vascular injury by preventing the contribution of the endothelium in proinflammatory and thrombogenic events. These agents inhibit the expression of molecules that participate in the activation of adhesion and transmigration of leukocytes, and improve vascular reactivity, preserving the vasodilation capacity by activation of K-ATP channels, reduction of intracellular calcium in smooth muscle vascular and nitric oxide release [6,8].
Ischemic preconditioning can decrease infarct size, lethal arrhythmia, and contractile dysfunction; similarly, the administration of anesthetics protects the myocardium from the effects of myocardial infarction and myocardial dysfunction. The dependent potassium adenosine triphosphate (ATP) channels play an important role; these channels in the myocardium are closed since ATP concentrations are elevated; When ischemia occurs and a state of hypoxia occurs, these channels open allowing the exit of potassium and loss of ATP, which generates the opening of the potassium channels of the mitochondrial membrane as a compensatory phenomenon, with the subsequent hyperpolarization of the cell membrane and decreased calcium influx and contractility. Halogenated agents induce a signal mediated by G proteins, which activate protein kinase C or PKC. PKC is also activated by phospholipids, reactive oxygen species and nitric oxide, it generates a signal that is transmitted to the effectors, in the channels of the mitochondria and the sarcoplasm. This will cause vasodilation in the smooth muscle of the coronary vessels, which will improve oxygen supply, and to a certain extent protects during ischemia when ATP production is reduced. It is believed that the mechanisms that participate in the cardioprotection processes are mainly those related to the preservation of mitochondrial function. By producing the reduction in intracellular calcium overload, it improves the preservation of energy reserves and prevention of the activation of promoting effects of apoptosis and cell necrosis [9-12] (Figure 1).
Figure 1: A review of myocardial ischaemia/reperfusion injury: Pathophysiology, experimental models, biomarkers, genetics and pharmacological treatment.2020.
Adenosine and Anesthetic Cardioprotection
Adenosine signaling participates in isoflurane-mediated cardioprotection, acting through four receptors designated as A1, A2a, A2b, and A3. Adenosine is the product of the conversion of AMP; in a study by Diamela et al. where it was evaluated whether remote ischemic preconditioning (rIPC) activates adenosine A1 receptors and reduces the size of the myocardial infarction, rat hearts were isolated exposing them to 30 minutes of ischemia and 60 minutes of reperfusion, while in another group of rats , a rIPC protocol was carried out, which showed that it reduces the size of the myocardial infarction by activating the A1 adenosine receptor at the beginning of myocardial reperfusion. This protective effect would also be mediated by the activation of the nitric oxide synthase enzyme during reperfusion [11,13,14].
Conclusion
Inhaled anesthetics protect against ischemia-reperfusion injuries. This is a phenomenon that occurs when cellular damage occurs secondary to the restoration of blood flow. Anesthetic preconditioning is the mechanism by which these drugs protect the myocardium from the effects induced by hypoxia and subsequent myocardial dysfunction; producing a cellular adaptation that avoids further injury to the tissue. The decrease in contractility and energy consumption by the cardiomyocyte allows the fibers to contract with less effort and generates a reversible involvement that prevents tissue necrosis and an increase in the affected area.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.