Infection of Piglets with the Porcine Respiratory and Reproductive Syndrome Virus (PRRSV): A Morphological Study
Introduction
The porcine respiratory and reproduction syndrome virus (PRRSV) forms small, enveloped particles (50-65 nm in diameter) harboring a relatively long (approximately 15 kb in size) single strand RNA genome [2]. The viral RNA (vRNA) is a positive-sense molecule with terminal cap at 5´-end and a poly-A repeat at 3´- end [3]. In the course of virus replication, the vRNA is copied as whole, when synthesized via a full length negative-strand RNA intermediate. The vRNA sequence begins with 2 (two) long open reading frames (called ORF1a and ORF1b), which together comprise about 75% of the total genome sequence [4]. This portion of the genome specifies 14 non-structural proteins (nsps) which are formed by cleavage of both translated polyproteins. Of special importance are, for example, two non-structural proteins (nsp9 and nsp12), which function as vRNA replicase, also termed RNAdependent RNA polymerase (RdRp) [5]. The rest of the genome encodes 7 structural proteins, out of which 5 are glycoproteins (designated GP2a/Gp2, GP2b/E, GP3, GP4 and GP5) along with the M (membrane) protein and the N nucleoprotein [6].
Regarding to the structure of the vRNA, the PRRSV has been classified as a member of family Arteriviridae (order Nidovirales), along with the equine arteriitis virus and the lactate dehydrogenase elevating virus of mice [7]. In the course of vRNA replication, a total length (genomic) minus strand is generated, which serves as template for the synthesis of new vRNA molecules. During viral mRNA synthesis, the negative sense RNA sequence is being formed first; then a set of positive sense nested sub genomic (sg) RNA molecules is transcribed. Finally, the full set of minus sense sub genomic (sg) RNAs is formed, which becomes a template for the synthesis of functional positive sense sg mRNAs [7]. Both strands are complementary to each other; the coterminal 3´-ends are equipped with a common leader sequence at their 5´-ends [8,9]. The viral genome reveals several (but at least two) conserved transcription regulatory sequences (TRS), which are located either in the front of ORF1a (encoding the structural protein GP2a) or before ORF2a (encoding the envelope glycoprotein Gp2b/E).
The classical PRRSV strains which were isolated in the US (VR2332) and/or in Europe (Lelystadt) differ at both, by serological as well as genome examinations [10]. Experimental infection with the PRRSV isolates can be lethal in newborn and/ or 3-week-old piglets. A key event of the infection process is the involvement of porcine alveolar macrophages, which are the most important virus target also mediating virus spread [11]. To date, at least two macrophage surface molecules are known as entry mediators: the siglec sialoadhesin and a scavenger receptor CD163 [12]. The PRRSV induced pneumonia is characterized by thickening of inter-alveolar septa due to infiltration with macrophages and by the presence of occasional inflammation and cell debris within the alveoli itself [13]. Also, alveolar pneumocytes of type II may be found PRRSV antigen positive along with the hyperplasia of peribronchial lymphatic tissue [14]. The severity of lung lesions may vary from relatively mild to quite extensive. The viral genotypes can differ in their pathogenicity, namely the Type 2 North American PRRSV induces more severe respiratory disease than type 1 European virus. Nevertheless, mild thickening of interalveolar septum can be mistaken with focal thickening of inter-alveolar septa in combination with slight infiltration of peri-bronchial connective tissue (referred to as mild non-specific interstitial infiltrate, MNSII), was occasionally seen in a proportion of non-infected control piglets and interpreted as unrelated to PRRSV infection [15]. In this paper we describe the correlation of the lung lesions as seen at histological examination slides stained with, HE on comparison to the immunohistochemical detection of viral N-protein along with the results of serological tests for N-protein antibodies.
Materials and Methods
Virus
A North American strain was cultured on the MARC-145 cell line; its titer end point (TCID50) was evaluated using a 96-well plate as described by Zhao, et al. [16] and/or Ramakrishnan (2016) [17].
Animals
Pigs (28 infected animals) were inoculated into both nostrils with 105 TCID50 of above mentioned PRRSV strain administered in a volume of 300μl culture supernatant. The negative control (9) animals were inoculated with a virus free culture medium; these animals were kept under conditions of careful isolation avoiding any contact with the virus-inoculated piglets.
Specimen Sampling and Histological Examination
At given intervals post-infection, the animals were succumbed. Blood was drawn for obtaining serum, whole the tissue samples (coming from each lung lobe, from both tonsils including adjacent paryngeal area, from spleen and liver) were removed and immediately immersed into in 10% neutral formalin for 24hr. Fixed tissue samples were rinsed in phosphate buffer, dehydrated in a series of corresponding reagents and embedded into paraffin as described Szeredi, et al. [18] and/or Stipkovits, et al. [19]. Next to sectioning, the sections were stained either by classical hematoxylin and eosin (HE) and/or treated by immunohistochemical reagents, namely using a commercial anti-PRRSV N-protein antibody mixture SDOW-17 and SR-30 in the first layer. This has been purchased from 4rtilab (SDOW17-A and SR30-A, respectively) and mixed for use in an equal 1:1 ratio.
ELISA Titer Measurements
The specific serum class IgG antibody levels against the PRRSV N antigen were determined using the INgezim PRRS 2.0 ELISA kit (purchaed from Eurofins) strictly following the procedure recommended by the manufacturer.
Saliva Collection
The pooled oral fluid was collected from each animal separately using the Civtest suis oral fluid rope IDEXX. Obtained saliva samples we examined for the presence of class IgA specific antibody to the N-antigen of PRRSV. The antibody test was performed with the Oral Fluids (IDEXX PRRS OF) kit as recommended in the manufacturer’ s manual.
Results
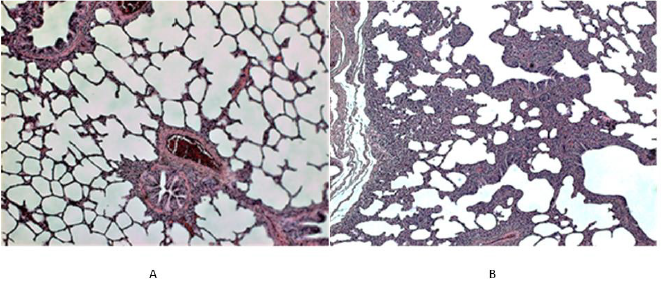
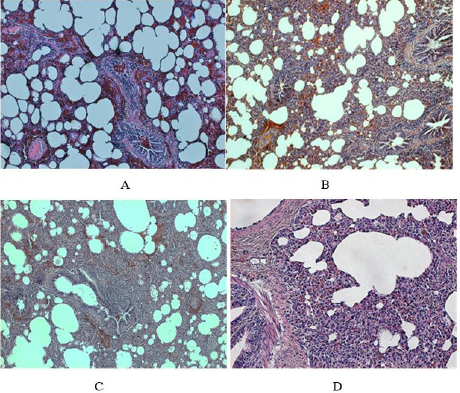
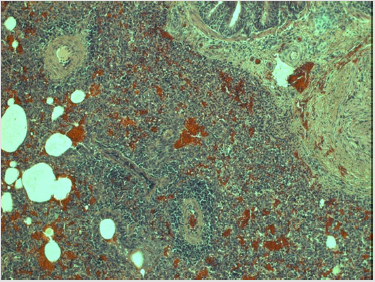
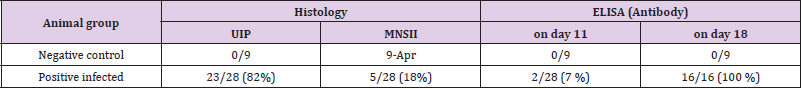
As documented in Figure 1A the interalveolar septi in normal lung tissue are very thin in order to ensure the diffusion of oxygen into blood capillaries, where the erythrocytes circulate. Occasionally, a few mononuclear cells (mainly lymphocytes) might be seen in the peribronchial connective tissue. Surprisingly, in 4 out of 9 uninfected (control) piglets, a slight focal thickening of interalveolar septi was noted along with the accumulation of relatively few interstitial infiltrates consisting of mononuclear cells, mainly lymphocytes (Figure 1B). As expected, staining for the N-antigen of PRRSV in the control lung tissue was negative by all control animals, including the above-mentioned areas in which the above mentioned mild interstitial infiltrate (MNSII) has been detected. The non-extensive MNSII (Figure 2A) has been also found in a few infected piglets (5 out of 28, 18 %), especially when the N-protein could not be detected (Table 1). As expected, in the majority of infected animals (23 out of 28) the lung tissue revealed a typical picture of usual interstitial pneumonia (UIP). In the latter, the interalveolar septi were thicker due to the presence of a rich mononuclear cell infiltrate. In UIP cases, the capillaries were widened along with occasional focal bleeding in result to the injury of endothelial cells (Figures 2B & 2C). High power view of such areas showed that the interstitial infiltrate in question consisted mainly of lymphocytes (Figure 2D). Occasionally (for example in piglet no. 40) the infiltrate was so extensive that it altered the original lung structure (Figure 3).
Figure 1: Histological picture of the lung tissue in uninfected (control) piglets.
A. In the left (piglet no. 5). The normal lung structure at low power view shows thin interalveolar septa devoid of any infiltrate; in the peribronchial (and/or perivascular) connective tissue a few mononuclear cells (mainly lymphocytes) can be seen.
B. In the right (piglet no. 2). Unlike to 1A, this Figure shows areas of thickened interalveolar septa due to the accumulation of monocellular cells (mainly of lymphocytes). Such focal mild non-specific interstitial infiltrate (MNSII) was found in the lungs of 5 out of 9 uninfected controls (Table 1).
Table 1: Survey of histological lesions and the N-antigen presence in infected piglets.
Note: *Mild non-specific interstitial infiltrate (in the peribronchial area and/or interalveolar septa); ** Severe interstitial infiltrate corresponding to the diagnosis of “Usual Interstitial Pneumonia” [1].
Figure 2: Histological findings in the lungs of PRRSV infected piglets.
A. In the left above (piglet no. 16). At low power view some areas of the lung tissue even in the infected animal showed rather less extensive thickening of interalveolar septa (infiltration by mononuclear cells referred to as mild non-specific interstitial infiltrate, MNSII); note the dilatation of small vessels (magn x100).
B. In the right above (piglet no. 16). In contrast to the area shown above, another lung area of the same reveals typical UIP with more widespread thickening of interalveolar septa and their abundant mononuclear cell infiltration (along with hyperemia, i.e. dilatation of capillaries and small blood vessels).
C. In the left below (piglet no. 25). The lung tissue of an animal who developed typical UIP shows widespread mononuclear infiltration of interalveolar septa and peribronchial connective tissue (a lymphatic follicle like structure can be seen, magn. x100).
D. In the right below, the same piglet as above (no. 25). The mononuclear infiltrate in the peribronchial area consists mainly of lymphocytes along causes thickening of interalveolar septa (magn. x240).
Figure 3: Extensive interstitial pneumonia in PRRSV infection.
Note: Lungs of the piglet no. 40 show severe interstial pneumonitis: the infiltration of interalveolar septa by mononuclear cells (mainly lymphocytes) is so widespread that the original lung structure can be hardly seen. In addition, the abundant hyperemia along with extensive proliferation of connective tissue is clearly visible (magn. 220x).
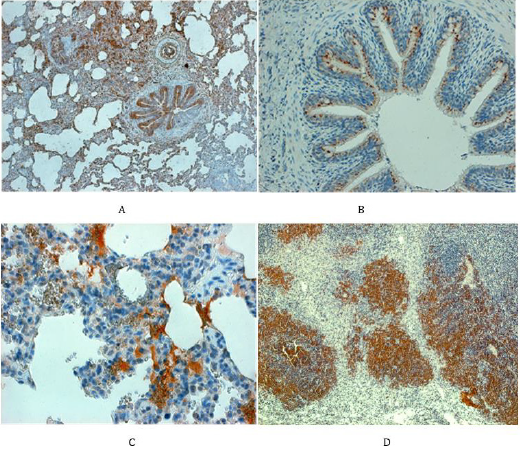
Staining with the anti-N antibody showed the presence of PRRSV antigen predominantly in the columnar ciliary epithelium lining the bronchial tree (Figure 4A). Details from such areas also demonstrated the presence of viral antigen in the cytoplasm of the small acinary mucous glands situated below the ciliary epithelium lining, namely in the connective tissue of bronchial wall (Figure 4B). The alveolar lining was rarely positive, though occasionally the type II alveolar cells could harbor the N-protein, mainly present in alveolar macrophages moving from the alveolar space across the thickened interalveolar septa into local lymphatic capillaries and/ or to the sinuses of regional lymph nodes (Figure 4C), where virus was finally deposited. Nevertheless, in some piglets the local lymph nodes did not stop the virus spread, which then might reach the spleen and/or liver via blood stream. The reticular cells of regional sinuses in spleen were found positive as well, and occasionally the antigen could be seen also in lymphatic follicles (Figure 4D).
Figure 4: Staining for N-antigen in the respiratory pathway and spleen
A. In the left above (piglet no. 34). The lungs of infected animals reveal overwhelming positive staining for N-protein, namely in the bronchial epithelium, in parabronchial mucinous glands and occasionally in the flat epithelium cells lining the aveoli (magn. 80x).
B. In the right above (piglet no. 12). The N-protein can be seen in the cytoplasm of ciliary epithelium cells lining the bronchi along with the negative goblet cells (magn. 120x).
C. In the left below (piglet no. 45). The N-protein can be seen in the cytoplasm of cells lining the alveolar wall and in mononuclear phagocytes which infiltrate the interalveolar septi (magn. 400x).
D. In the right below (animal no. 44): the spleen showing lymphatic follicles consisting mainly of lymphocytes positive for the N-protein (magn.x120).
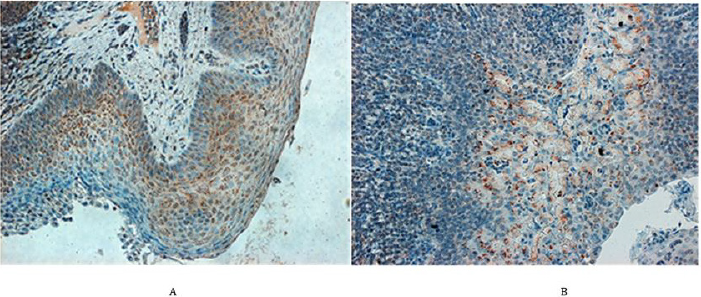
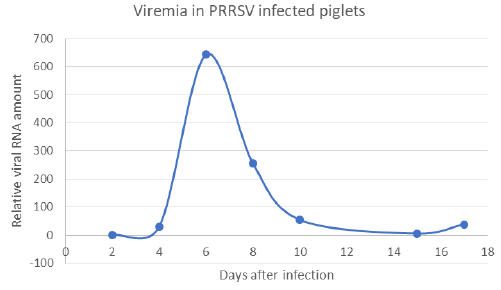
Outside of lung tissue, the N-protein of PRRSV was found especially in the non-hornified squamous epithelium of the pharyngeal area including that over tonsil (Figure 5A). Here the virus antigen occupied the deeper layers of stratified epithelium, namely the multiplying parabasal cells as well as those in the medium layer. The virus was also found in the salivary glands as documented in submandibular gland, which acinar cells harbored the N-antigen in their cytoplasm (Figure 5B). While the tonsils and/ or pharyngeal epithelium were involved relatively frequently, the presence of the virus in salivary glands acinar cell was relatively rare. Nevertheless, the real incidence of given antigen in the salivary glands was difficult to assess, since such tissue has appeared in the sections examined just by chance. The development of antibody response in comparison with lung lesions as detected by ELISA in serum samples is documented on Table 2. This shows that the virusspecific antibodies were rarely detected on day 11 post-infection but were frequently found on day 18. This may not be surprising and can be explained by the viremia (Figure 6), which peaked on day 6 post-infection, but was absent by day 10. Interestingly enough, on next day 11, the ELISA could not detect free serum antibodies, probably because they were bound to virus particles. However, the specific antibodies were clearly detected at later intervals (i.e. by day 18), when their levels in the serum increased.
Figure 5: N-protein in the pharyngeal area of PRRSV infected animals.
A. In the left (piglet no. 26). In the tonsillar squamous epithelium, the N-protein is expressed mainly wthin cytoplasm of actively growing cells of the suprabasal and intermedial layers including a few basal epithelium cells (magn. 220x) B. In the right (pig no. 13). N-protein can be seen in the acini of a submandibular salivary gland as well as in the marginal sinus of adjacent lymph node (magn. 220x).
Figure 6: Presence of vRNA in the serum of infected pigs (viremia has been detected on days 6 and 8 post-infection).
Table 2: The comparison of UIP with serological response.
Discussion
The PRR syndrome in piglets is characterized with high mortality, reproductive failure (late-term abortions and stillbirths, premature farrowing, mummified pigs in pregnant sows) and a severe respiratory disease (interstitial pneumonia). The disease occurring in the nursery and among growing/finishing piglets causes significant economic losses to the swine industry worldwide. The corresponding virus (PRRSV) replicates mainly in the porcine alveolar macrophages (PAMs) and dendritic cells (DCs) [20]. The virus also causes persistent infection eliciting antibody dependent enhancement (ADE) and occasional immunosuppression. Being a member of the family Arteriviridae, it belongs to the order Nidovirales together with the Coronaviridae and Roniviridae families [21]. PRRSV was originally divided into European type 1 and North American type 2 genotypes. Later on, the East European PRRSV isolates have been found to be of the European genotype but forming different subtypes. A novel virus, namely the Belarusian strain Lena, has been recently characterized as a highly pathogenic East European subtype 3, which differs from European subtype 1 Lelystad and North American US5 strains at genetic as well as antigenic levels [22].
Numerous results suggest that PRRSV may utilize multiple strategies of replication and spread in the infected pigs, including subversion of the host innate immune response, inducing an antiapoptotic and anti-inflammatory state as well as developing ADE. The PRRSV induced immunosuppression might mediate apoptosis of infected cells, which causes depletion of immune cells and induces an anti-inflammatory cytokine response due to which the host is unable to eradicate the primary infection. The initial antibodies do not confer protection and can even be harmful by mediating an antibody-dependent enhancement (ADE), since they can facilitate the virus entry of into targets cells in vitro. To characterize the humoral immune response direct enzyme-linked immunosorbent assays (ELISA) can be used including different mainly recombinant PRRSV antigens. For example, the kinetics of antibody responses directed against nonstructural virus coded proteins (nsp) can be analysed in pigs experimentally exposed to the virus [23]. In such case, high antibody reactivities especially against nsp1, nsp2, and nsp7 were noted. Among the latter, nsp7 recombinant proteinbased ELISA showed good sensitivity and specificity most suitable for diagnostic development especially for identification and differentiation of type 1 and type 2 PRRSV. Several non-structural proteins (such as nsp1, nsp2, nsp5, nsp7 nsp9, nsp10 and nsp11) have been implicated in the induction of IFN-γ and also in the development of the cell-mediated immune response [24]. On other hand, the induction of neutralizing antibodies (NAs) may be delayed and/or their levels may remain low, which is not only the problem of early diagnostic, but is also of importance regarding effective virus elimination. NAs may protect against disease if present in sufficient quantities before infection, but they do not seem to be essential for clearing virus in blood during the course of the infection. PRRSV is able to modulate innate responses, probably through the regulation of IFN-α and IL-10 responses [25].
As described, PRRSV replicates predominantly in the lung alveolar macrophages, can induce prolonged viremia, and cause persistent infections lasting for months after initial infection. PRRSV strongly modulates the host’s immune response and changes its gene expression. Studies showed that PRRSV inhibits type I interferons (IFN-β). Regarding cell-mediated responses, development of PRRSV-specific gamma interferon-secreting cells (IFN gamma-SC) and interleukin 4-secreting cells (IL4-SC) in PBMC was examined by ELISPOT assay. Using this technic, no IFN gamma-SC was detected until day 14 p.i., whereas for IL4-SC, such differences were not seen. Concurrently with the onset of viremia and the development of clinical signs, serum haptoglobin levels and interleukin 10 (IL10) in PRRSV-stimulated PBMC-culture supernatants increased significantly. These results are compatible with the model of pathogenesis in which the immune response does not fully control the outcome of infection [26].
The PRRSV replication and its spread in the body subverts the host innate immune response as well when high jacking its lipid metabolism and inducing an anti-apoptotic and anti-inflammatory state. The latter is indicated by suppressing the expression of serine proteinase inhibitor 2 (SPI 2), IFN-α, and down-regulation of the expression of pro-apoptotic genes such as B-cell lymphoma 2 (BCL-2) antagonist/killer (BAK) and the BCL-2 associated X (BAX). Whereas BAX resides predominantly in the cytosol, BAK is constitutively localized to the outer mitochondrial membrane; both form toxic mitochondrial pores in response to cellular stress. Furthermore, the APR-1, i.e., the Adenomatous polyposis coli (APC) protein which is a Wnt signaling component along with a microtubule-associated protein SARP3 (several ankyrin repeat protein 3), may be downregulated. Both were shown to interact with all isoforms of PP1 (protein phosphatase 1). Infections of N-PRRSV viruses resulted in fever and inflammatory response, as indicated by high expression of proinflammatory cytokines and chemokines, adhesion molecules, inflammatory enzymes and their receptors, such as IL-1β, IL8, SELL, ICAM, CCL2, CXCL9, CXCL10, B2M, proteasomes and cathepsins. This was compounded by cell death and elevated expression of NFKBIA, XAF1, GADD45A, perforin, granzymes, and cytochrome C, coupled with increased ROS-mediated oxidative stress, as indicated by up-regulated expression of cytochrome b245. Taken together, the N-PRRSV infection may have resulted in an excessive immune and inflammatory response that contributed to tissue damage [27].




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.