Methods for Determining the Energy Function of Mitochondria
Introduction
Energy exchange in the cell is associated with mitochondria, which play an important role in vital processes, participating not only in the formation of ATP but also in the storage and transmission of hereditary information, apoptosis and plastic processes [1,2]. Mitochondria are very mobile and plastic organelles that constantly change their shape, merge, and then separate again. The movement of mitochondria in the cytoplasm is associated with microtubules, which determines their orientation and distribution in the cell. In some cells, mitochondria form long mobile filaments or chains, while in others, they are fixed near the places of consumption of ATP [3,4]. Each mitochondrion contains highly specialized membranes that play a key rolein its activity. The membranes form two isolated mitochondrial compartments: the inner matrix and the narrow intermembrane space. Each section contains a unique set of proteins [5,2]. The outer membrane contains the protein porin, which forms wide hydrophilic channels in the lipid bilayer, resulting in a membrane-like sieve, permeable to all molecules weighing less than 10,000 daltons.
These molecules can penetrate the intermembrane space, but most of them are unable to pass through the impermeable inner membrane [1,6]. The main functional part of mitochondria is the matrix and the surrounding inner membrane. The matrix of mitochondria has a more viscous consistency than the cytoplasm of the cell. It contains enzymes, mitochondrial DNA, ribosomes, organic compounds, ions, including calcium and magnesium. Matrix enzymes are involved in the Krebs cycle, oxidative phosphorylation, pyruvate oxidation, and beta-oxidation of fatty acids [2]. The inner membrane forms a complex system of folds in the mitochondrial matrix – cristae, which significantly increase its area. For mitochondrial cristae in cells of various organs, morphological features and different enzymatic compositions are characteristic [7]. The most characteristic feature of organelles that the presence of enzyme complexes involved in oxidative phosphorylation and energy supply to the cell.
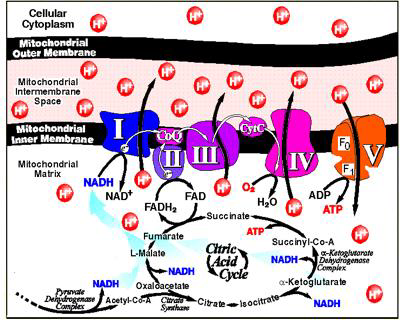
Most of the enzyme proteins are components of the electron transport chain that maintains a proton gradient across the membrane. Another large protein complex is the enzyme ATP synthase, which catalyzes the synthesis of ATP [8]. In mitochondria, oxidative metabolism takes place, the substrate for which is mainly fatty acids and pyruvate, formed as a result of glycolysis in the cytosol. These substances are transported from the cytosol to the mitochondrial matrix, where they break down into twocarbon groups, combined with acetyl coenzyme A (acetyl-CoA). In the composition of the acetyl-CoA molecule, each acetyl group is included in the Krebs cycle as a source of high-energy electrons. Electrons are transferred to the respiratory chain of the inner mitochondrial membrane, where energy is generated as a result of their transfer (Figure 1).
Figure 1: Complexes of the mitochondrial respiratory chain.
Note: complex I (NADH-ubiquinone oxidoreductase; NADH-dehydrogenase), complex II (succinate dehydrogenase; succinate-ubiquinone reductase), complex III (cytochrome bc1 complex; ubiquinone-cytochrome c oxidoreductase, complex IV (cytochrome c oxidase, complex V (mitochondrial ATP synthase) [9]
Disorders of energy metabolism in the cell are one of the key links in many diseases. This makes it necessary to study the work of the electron transport chain of mitochondria, both of its complexes and the entire chain as a whole [9]. The purpose of this review is to analyze and systematize literature data on methodological approaches to the study of the energy function of mitochondria.The Oxidative Phosphorylation System (OxРhoS), localized in the inner mitochondrial membrane, consists of five membrane enzymes. Four of the five protein complexes make up the “respiratory chain” and are involved in the transfer of electrons, which at three points is coupled with the translocation of protons across the inner mitochondrial membrane. The resulting proton gradient is used by the ATP synthase complex (the fifth enzyme complex) to phosphorylate ADP [10,11,1]. For a long time, a fluid-state model has been used to describe the organization of the OxPhoS system. According to this model, the complexes of the respiratory chain freely diffuse in the membrane, and the transfer of electrons occurs as a result of random chaotic collisions.
This model is based on the fact that all protein complexes of the OxPhoS system can be isolated while maintaining enzymatic activity [8,9]. In the last decade, there is more and more evidence indicating stable interactions of OxPhoS complexes in the form of supercomplexes. It is assumed that the OxPhoS supercomplexes and their single complexes coexist in the inner mitochondrial membrane. The association of complexes into super-complexes and the dissociation of super-complexes into OxPhoS complexes is a dynamic process that dependson the physiological state of the cell. Recent studies of mitochondria show that ATP synthase in mitochondrial membranes is organized into long strips of dimers and mitochondrial cristae act as proton traps, and ATP synthase can optimize its activity when there is a lack of protons [12,13]. Complex I, or NADH dehydrogenase, is the main entry point for electrons into the respiratory chain. Complex I oxidizes NAD-H, taking two electrons and reducing one ubiquinone Q molecule, which is released in the membrane. Ubiquinone Q is lipid-soluble; inside the membrane, it diffuses to complex III.
Complex I plays a central role in cellular respiration and oxidative phosphorylation, providing up to 40% of the proton gradient for ATP synthesis. During the oxidation of one NADH molecule, the NADH-dehydrogenase complex transfers four H+ protons from the matrix to the intermembrane space of the mitochondria through the membrane. The formation of reduced NADH is associated with the conversion of malate to oxaloacetic acid and glutamate to α-ketoglutarate. The transfer of electrons through the I complex is associated with the release of 3 ATP molecules [14,1]. Complex II, or succinate dehydrogenase, is another entry point for electrons into the respiratory chain, it is not associated with the translocation of protons across the membrane, transfers electrons from succinate to ubiquinone, and directly binds the Krebs cycle with the respiratory chain. In this case, succinate is oxidized to fumarate with subsequent reduction of ubiquinone Q.
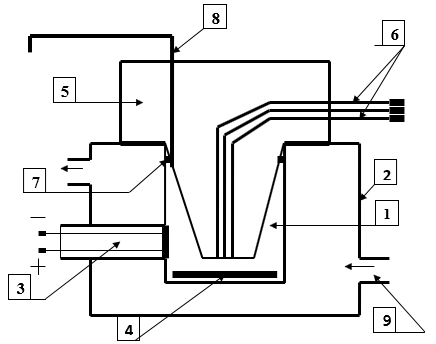
Electrons from succinate are first transferred to flavinadenine dinucleotide, and then through Fe-S clusters to ubiquinone Q. Electronic transport of the II complex is not accompanied by the creation of a proton gradient. The H + protons formed during the oxidation of succinate remain in the matrix and then are reabsorbed during the reduction of the quinone. Complex II works as a carrier of electrons with the formation of 2 ATP molecules [15,1].Respiration of mitochondria and the work of complexes I and II are assessed by registering the rate of oxygen consumption in a polarographic cell using a built-in Clarke electrode (volume 3 ml, at a temperature of 25 ° C (Figure 2).To study the operation of complexes I and II, the rat brain is removed in the cold (0-4 ° C), dried with filter paper, and homogenized in an isolation medium (0.32 M sucrose, 10 mM Tris-HCl, 1 mM ethylenediaminetetraacetic acid, pH 7.4 in the ratio 1:10) using a Potter-Evelheim homogenizer with a Teflon pestle according to a modified method [16,17].
Figure 2: Polarographic cell for studying the respiratory activity of mitochondria
1. Cell
2. Thermostatically controlled chamber
3. Clarke electrode
4. Magnetic stirrer
5. Sealing plug
6. Channels for dosed anaerobic administration of substrates and ADP
7. A sealing ring
8. Channel for removing air and excess liquid
9. Fitting for connection to an ultrathermostat.
Mitochondria are isolated by differential centrifugation. The nuclear fraction is separated by centrifugation of the brain homogenate at 600 g for 10 min (4 ° C). The resulting supernatant is centrifuged at 8500 g for 10 min (4 ° C), the mitochondrial pellet is washed twice in the isolation medium and resuspended to a protein concentration of 35-40 mg/ml in the isolation medium and stored in a short tube on ice. Protein concentration is determined by the Lowry method [16]. A concentrated suspension of mitochondria is introduced into a thermostatted sealed polarographic cell with an incubation medium (0.17 M sucrose, 40 mM KCl, 10 mM Tris-HCl, 5 mM KH2PO4, 8 mM KHCO3, 0.1 mM ethylenediaminetetraacetic acid, pH 7.4) in an amount providing a final protein concentration in the cell of 1 mg/ml. Registration of changes in oxygen tension (pO2) in the mitochondrial suspension is carried out using an electronic recorder. The Clarke electrode is calibrated by sequentially blowing ai) and gaseous nitrogen through the cell.
Basal respiration is assessed, as well as respiration stimulated by the introduction of substrates: malate/glutamate to assess the work of complex I and succinate to assess the work of complex II. The following indicators of mitochondrial respiration are recorded: V1 - basal respiration rate, V2 - substrate-dependent respiration rate, V3 - respiration rate associated with phosphorylation (after ADP introduction), V4 - respiration rate after completion of added ADP phosphorylation. Indicators characterizing the conjugation of oxidation and phosphorylation processes in mitochondria are determined: the acceptor control coefficient (V3 / V2), the respiratory control coefficient (V3 / V4), and the phosphorylation coefficient - ADP / O. After recording the rate of basal (endogenous) respiration in the absence of a substrate (V1), respiration substrates (malate - 2 mM/glutamate - 5 mM or succinate - 5 mM) are alternately introduced into the mitochondrial suspension, and then ADP in an amount of 200 nmol/ml.
The obtained polarograms are used to calculate the respiration rate of mitochondria in different metabolic states and the coefficients characterizing the conjugation of oxidation and phosphorylation processes. The use of solutions of substrates of succinate and malate/glutamate complex makes it possible to assess the degree of functional activity of complexes I and II of the electron transport chain [16]. The central component of the OxPhoS system is cytochrome c-reductase, or complex III, which functions as a dimer. It transports electrons from reduced ubiquinone (ubiquinol) to cytochrome c, a small mobile electron carrier bound to the outer surface of the inner membrane. This multiprotein transmembrane complex is encoded by the mitochondrial (cytochrome b) and nuclear genomes [18]. Electronic transport in complex III is associated with the transfer of protons from the matrix to the intermembrane space and the generation of a proton gradient on the mitochondrial membrane. Cytochrome c is a component of the electron transport chain, the function of which is to transfer electrons between complex III (ubiquinonecytochrome c-reductase or cytochrome bc complex) and complex IV (cytochrome c-oxidase).
For every two electrons passing along the chain of transfer from ubiquinone to cytochrome c, two photons are absorbed from the matrix, and four more protons are released into the intermembrane space. The reduced cytochrome c moves along the membrane in an aqueous medium and transfers one electron to the next respiratory complex, cytochrome oxidase [19,9]. It is the only peripheral protein that interacts with the outside of the inner mitochondrial membrane.Cytochrome c is a water-soluble protein of low molecular weight (about 12,000 Da), the primary structure of which contains about 100 amino acids. Cytochrome c can catalyze hydroxylation and aromatic oxidation reactions and has peroxidase activity by oxidizing various electron donors [20]. Cytochrome c is a metalloprotein that functions in electron transfer reactions and contains heme c (or several hemes) as a prosthetic group, covalently bound to a protein molecule through one or two thioether bonds between the cofactor and the sulfhydryl group of cysteine squirrel. The ligand in the 5th coordination position of the iron ion is always histidine.
Cytochrome c is localized in the intermembrane space. All cytochromes c can be divided into four classes [10].The first class is the low-spin form of soluble cytochrome c of mitochondria and bacteria, in which histidine bound to the heme is located in the N-terminal part of the protein molecule, and the ligand in the 6th coordination position - methionine is located 40 residues towards The C-terminus of the molecule [18].The second class is the high-spin form of cytochrome c and several low-spin forms (for example, cytochrome c 556), which have heme-binding sites in the C-terminal region of the protein molecule. The protein contains four helical regions of the polypeptide chain [19]. The third class includes cytochromes containing several hemes and having a low redox potential (cytochrome c7 (three hemes), c3 (four hemes), and high molecular weight cytochrome c (hexadecahaem)), in which there are 30-40 residues per heme molecule. Hemes, coordinated by two histidines, are structurally and functionally nonequivalent and are characterized by different redox potentials from 0 to 400 mV [19,18].
The fourth class is necessary to maintain complex proteins with heme and other prosthetic groups, for example, flavocytochrome c, cytochromes cd. Cytochromes of this class are proteins containing four hemes, which in the 5th and 6th coordination positions have either two histidines or histidine and methionine. Mitochondrial cytochrome c is one of the three redox subunits of the third complex of the respiratory chain (cytochrome bc complex) [11,19]. Mitochondrial cytochrome c is anchored in the membrane by one membrane segment near the C-terminus. A water-soluble cytochrome C1 preparation can be obtained by removing the hydrophobic C-terminal region. Water-soluble cytochrome c1 cannot participate in the assembly of the bc complex [11,18]. Cytochrome c, in addition to the function of a carrier of electrons in the respiratory chain, can separate from the inner mitochondrial membrane, be transported into the cytoplasm of the cell, and trigger a chain of events in the cytosol, which accelerates apoptosis [11].
The ability of cytochrome c to exhibit various functions inside mitochondria and in the cytosol is associated with the cellular localization of the hemoprotein. Cytochromes are an important class of metal proteins involved in electron transfer and redox catalysis. Redox enzymes and metal proteins play an important role in signaling processes, are responsible for the regulation of genes and their expression, provide the conversion of energy in the processes of respiration and photosynthesis. Thus, cytochrome c in the electron transport chain of mitochondria acts as an electron acceptor for complex III (cytochrome c reductase) and an electron donor for complex IV (cytochrome oxidase) [20,21]. Determination of the activity of complex III The activity of complex III is determined spectrophotometrically at a wavelength of 550 nm by antimycin A-sensitive reduction of ferri- and ferrocytochrome with decylubiquinone in the presence of Tween 20, albumin, and sodium azide.To measure the activity of complex III, the mitochondrial suspension is diluted to a concentration of 0.05 mg/ml of protein in a medium containing 35 mM KH2PO4, 2 mM NaCN, 0.5 mM ethylenediaminetetraacetic acid, pH 7.25. The samples are then sonicated in a water bath for 30 s.
The activity of the complex is determined by the rate of antimycin-sensitive reduction of cytochrome c (550 nm, molar extinction coefficient 18,500 M4 cm4) with the addition of 60 μM decilubiquinone, 50 μM cytochrome c, and 5 mM MgCl2 to a suspension of mitochondria exposed to ultrasound (the content of mitochondrial protein in the sample is 0.01 mg/ ml). Spectrophotometric determination of the concentration of cytochromes c and b in aqueous solutions. The molar extinction coefficients for cytochrome c A vost-oxide = 18,500 M4 cm-1 at 550 nm. Recovery is carried out with ascorbate or dithionite. In the presence of cytochrome b, the content of cytochrome c was determined from the differential spectra of the forms reduced by ascorbate, minus the forms oxidized by ferricyanide. Cytochrome b is determined from the differential spectra of the forms reduced by dithionite minus the spectra of the forms reduced by ascorbate. The activity of enzyme systems seems to be the most important parameter characterizing the work of the electron transport chain of mitochondria and the bioenergetic status of the cell.
Extraction of cytochrome c from the mitochondrial membrane and the reconstruction of the respiratory chain makes it possible to assess the transfer of electrons from complex III to IV. The principle of the method for extracting cytochrome c from the mitochondrial membrane consists of the destruction of the outer membrane using detergents or hypotonic processing and extraction of proteins with saline solutions. Reagents for isolation of mitochondria include: 0.25 M sucrose solution, 0.15 and 0.015 M KS solutions, 5 mM potassium succinate solution, pH 7.4, 5 mM potassium glutamate solution, pH 7.4, 0.005 M solution 2.4 dinitrophenol, pH 7.4, incubation medium (0.15 M sucrose, 0.075 M KC1, 0.01 M potassium phosphate, pH 7.4), cytochrome c solution - 1 mg / ml and reagents for protein determination. All solutions are prepared with bidistilled water. Isolated mitochondria are suspended in 3 ml of 0.25 M sucrose solution. Three conical flasks are filled with 40 ml of solutions:
1) 0.25 M sucrose;
2) 0.15 M KC1
3) 0.015 M KC1. 1 ml of a thick suspension of mitochondria is added to each flask. The contents of all flasks are gently mixed for 10 minutes at 0 ° C and then transferred to three 50 ml centrifuge beakers.
The mitochondria are separated by centrifugation at 10,000 g, suspended in 0.5 ml of 0.15 M KC1, and transferred into three flasks containing 40 ml of 0.15 M KC1. The contents of the flasks are again stirred in the cold for 10 minutes and centrifuged again under the same conditions to separate the mitochondria. The resulting sediments of mitochondria are resuspended in 0.3-0.5 ml of 0.3 M sucrose solution. The rates of oxygen consumption are determined polarographically, using succinate and glutamate (5 mM) as substrates, and after 1-2 minutes dinitrophenol (50-100 μM) is added. For a preparation washed with a hypotonic solution of KCl, the dependence of the rate of succinate oxidation on the amount of cytochrome c added to the incubation medium (concentration from 0 to 100 μg / 2 ml, 5-6 points) is determined [15,4 ,11]. Complex IV, or cytochrome c oxidase, is the final complex of the respiratory chain. Cytochrome c oxidase catalyzes the transfer of electrons from cytochrome c to molecular oxygen, reducing the latter to water.
Complex IV is the terminal oxidase of the aerobic respiratory electron transport chain, which catalyzes the transfer of electrons from cytochrome c to oxygen to form water. Complex IV sequentially oxidizes four cytochrome c molecules and, accepting four electrons, reduces O2 to H2O. During O2 reduction, four H+ are captured from the mitochondrial matrix to form two H2O molecules, and four more H+ are actively pumped across the membrane. Thus, cytochrome oxidase contributes to the creation of a proton gradient for ATP synthesis and is part of the oxidative phosphorylation pathway [11,4]. Complex IV activity Cytochrome oxidase activity is assessed by the polarographic method according to the rate of oxygen consumption by mitochondria. It should be noted that all procedures for the isolation, operation, and storage of mitochondria must be carried out observing the temperature regime: the samples must be stored in ice, the media and the instrument must be pre-cooled to a temperature of 0-4 ° C. A suspension of intact mitochondria or mitochondria destroyed by detergent is introduced into a polarograph cell containing a medium of the following composition: 0.125 M sucrose, 60 mM KCl, 10 mM Tris-HCl, 0.1 mM 2,4-dinitrophenol, 40 μM cytochrome c.
The content of mitochondrial protein in a polarographic cell is 0.5-1.0 mg/ml. The cytochrome oxidase reaction is started by introducing a solution of ascorbic acid neutralized to pH 7.4, creating a concentration of 10 mM in the sample. Measure the rate of oxygen uptake and calculate the activity of cytochrome oxidase in nanomoles of absorbed 02 for 1 min per 1 mg of protein [17]. ATP synthase, complex V, uses the resulting proton gradient to synthesize ATP. ATP synthase is an integral protein of the inner mitochondrial membrane that carries out the reaction of ATP formation from ADP [20]. Mitochondrial ATP synthase plays an important role in stem cell differentiation, promotes the maturation of mitochondrial cristae by dimerization and specific regulation. The enzyme belongs to the alpha/beta ATP synthase family. It consists of two structural domains (F1 - extramembrane catalyst and F0 - membrane proton channel), connected by a central rod, consisting of γ, δ, and ε subunits, and together with the membrane subunit oligomer representing the rotary domain of the enzyme [15].
Determination of the content of ATP synthase (V complex) is carried out by the immunohistochemical method using monoclonal antibodies. For this purpose, after decapitation and extraction of the brain, the material is fixed in zinc-ethanol-formaldehyde at + 4 ° C (overnight), then embedded in paraffin [22]. Paraffin sections with a thickness of 5 μm are prepared using a microtome, mounted on glass slides. The preparations are processed according to the protocol of immunocytochemical reaction for light microscopy, excluding the procedure of thermal unmasking of antigens. To determine the immunoreactivity of the molecular marker of mitochondria ATP synthase (complex V, which forms ATP from ADP), primary monoclonal antibodies (Anti-ATP5A antibody) are used at a dilution of 1: 2400 at + 4 ° C, with an exposure of 20 h in a humid chamber [22]. Thus, the above methods for studying the activity of the electron transport chain of mitochondria, especially their use in combination, can significantly detail the understanding of the pathogenesis of disorders of cell energy exchange that occurs in various diseases, which will improve the prevention and correction of mitochondrial dysfunction.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.