The NRH: Quinone Oxidoreductase Enzyme (QR2) has a Particular Distribution in Avian Retina
Introduction
The cytosolic detoxifying enzyme Ribosyldihidronicotinamide dehydrogenase (quinone), EC 1.10.99.2 (NRH: Quinone Reductase, QR2) is among the phase II detoxifying enzymes. Mice lack QR2 presents reduced sensitivity to menadione and myeloid hyperplasia of the bone marrow in comparison with the wild mice, proving that this enzyme is naturally active [1]. Regarding QR2 substrate, the para-quinone menadione is the most used in in vitro assays [2], but ortho-quinones such as dopamine quinone and estrogen quinone appear to be preferentially QR2 substrates in activity assays in vitro [3-5] and in situ [4,6]. The ability of the QR2 to retrieve dopamine from dopamine quinone is important in the retina because dopamine is the main modulator of the light-adapting events that are related to forming-image vision, besides dopamine has others trophic and circadian effects [7]. Therefore, a little change in dopamine levels can alter the retinal physiology.
In the retina, dopamine synthesis is triggered by light [4] and inhibited by melatonin [8]. The rate-limiting enzyme for dopamine synthesis is tyrosine hydroxylase, EC 1.14.16.2 (TH), which converts tyrosine to L-dopa, which is the substrate of the Aromatic L-amino acid decarboxylase, EC 4.1.1.28 (DOPA decarboxylase) in dopamine synthesis. DOPA decarboxylase and TH are in the type I amacrine cells, but in chick retina DOPA decarboxylase was also located in the type II amacrine cells and in photoreceptors [9]. The dopamine extracellular cleaner is done by the selective dopamine transporters that are located in dopaminergic amacrine cells [10]. However, a recent study in mammalian shows that the dopamine uptake and re-uptake also occur by non-selective transporters located in ganglion cells, in horizontal cells, in photoreceptors and in retinal pigment epithelial cells [11]. An interesting QR2 ligand is melatonin, which is the neurohormone produced in the dark phase of the circadian rhythm, mainly in the pineal gland and in the retinal photoreceptors [12,13]. Melatonin is the main modulator of the dark-adapting events in the retinal physiology [14]. The rate limiting enzyme in the melatonin synthesis is the Arylalkylamine N-acetyltransferase, (AANAT, EC 2.3.1.87), which is self-controlled by local molecular clocks and inhibited by dopamine [15]. Regarding non-neural retina, human retinal pigment epithelial cells in culture express functional melatonin synthesis machinery [16].
Melatonin has at least two binding modes in the QR2 pocket. The most common binding mode is that where melatonin is in parallel with FAD cofactor, matching well with the position of the inhibitors in the QR2 active site [17,18]. This observation is in accord with melatonin inhibiting QR2 in in vitro studies [19], suggesting that the same behaviour occurs in situ. On the other hand, the melatonin role in the QR2 activity in situ is very difficult to ascertain first because melatonin has membrane receptors and other binding sites in the retina [20], and second because the melatonin behaviour in the QR2 catalyses is dependent on its binding mode into QR2 active site [21]. In the chick retina, our previously published results showed that the differentiation of the retinal cells in culture is not completed in the absence of the melatonin [21,22]. Melatonin action on membrane receptors results in the inhibition of the cAMP accumulation [23], while the accumulation of this nucleotide is increased in this tissue by incubation with 5-MCA-NAT, which is a melatonin analogue and QR2 ligand [9]. The rising in cAMP levels by 5-MCA-NAT is a consequence of the QR2 recovering endogenous dopamine in developing and early PH retinas. In fact, the endogenous dopamine levels are increased by 5-MCA-NAT and by the QR2 cosubstrate N-methyl-dihydronicotinamide, while the QR2 inhibitor benzo[e] pyrene inhibits the endogenous dopamine levels in both control and stimulate retinas [6]. Regarding morphological data about QR2 in the retina, dense immunoreactivity for this enzyme has been shown in the whole neural retinas of the embryonic and early PH chicks [24] and in the whole neural retinas of the developing and juvenile turtles [18]. Thus, the current morphological studies do not show the QR2 distribution in the retinal layers. For that reason, these investigations offer limited insights concerning both the role of the melatonin into the QR2 binding site and the QR2 dopamine quinone activity in situ. As never was accomplished a study showing the distribution of the QR2 in the retina, the present aim was to investigate by immunohistochemistry the localization of the QR2 in retinal layers of the early PH chicks.
Material and Methods
Experimental Animals
Three early (3-5 day old) post-hatched (PH) chicks (male and female) were maintained at the equatorial photoperiod (12 h light/ 12 h dark, without annual variation) and sacrificed by decapitation in the morning. All possible efforts were made to avoid animal suffering and distress. All experimental procedures were carried out in accordance with the Brazilian laws for animal experiments, under license of the Ethics Committee on Experimental Animals of the Federal University of Pará (CEPAE-UFPA: BIO021-11).
Materials
NQO2 (N-15) polyclonal goat primary antibody (sc-18574) and chicken anti-goat Ig-G Texas Red (sc-3923) antibody were purchased from Santa Cruz Biotechnology Inc., CA, USA. Horse normal serum (S-2000), biotinylated horse anti-goat IgG antibody (BA-9500), Vectastain ABC Kit (PK-4005) and 3,3’Diaminobenzidine (DAB) peroxidase substrate (SK-4100) were purchased from Vector Laboratories, Burlingame, CA, USA. 4,6-Diamino-2-phenylindole dihydrochloride (DAPI) (D8417), Chicken serum (C5405) and Tween 20 (P2287) were purchased from Sigma Chemical Co., Saint Louis, MO, USA.
Tissue Preparation
Eyes of the PH chicks were enucleated and put in calcium magnesium free saline solution at 4°C. The anterior portion of the eye was removed. The cup containing the whole retina was immediately post-fixed for 24 hours in 4% paraformaldehyde, after the cup was put under different gradients of sucrose-glycerol solutions over 7 days. All solutions were at pH 7.4. The cup was frozen in Tissue Tek, and 30 μm [25] vertical and oblique sections were cut using a cryostat (Carl Zeiss Micron, Germany). An assortment of sections were mounted onto gelatinized slides and stored in a freezer at -20°C.
Immunohistochemistry
Texas Red Immunofluorescence: We used a commercial primary polyclonal goat antibody against avian QR2 enzyme, NQO2: N15 (sc-18574), previously validated for detection of the QR2 by immunofluorescence. The non-specific binding of the secondary immunofluorescent antibody chicken anti-goat Ig-G Texas Red (sc-3923) was prevented by including normal chicken serum (0.2%) in the emulsifying solution performed with Tween 20 (0.5%) in PBS. Negative controls of the secondary antibody binding were performed incubating retinal slices for 24 h at 4°C in a PBS solution devoid of the QR2 primary antibody. All solutions were at pH 7.4. Sections were incubated in the PBS solution containing Tween 20 (0.5%) and chicken serum (0.2%) for 30 minutes at room temperature. Then, the sections were rinsed (PBS, 3 times, 10 minutes) and incubated in the PBS solution containing 1:250 NQO2 primary antibody, for 24 h at 4°C. The negative controls were incubated for 24 h at 4°C in a simple PBS solution. After incubation, all sections were washed (PBS, 3 times, 10 min) and incubated in the PBS solution containing 1:400 Texas Red secondary antibody (anti-goat, done in chicken) for 2 h at 4°C. The secondary antibody reaction was stopped by exhaustive wash with PBS solution in room temperature. In some sections, cell’s nucleus were counterstaining with 0.001% DAPI for 15 seconds. The mounting medium (ibidi GmbH, Martinsried, Germany) was applied in the sections that were covered and analysed using a Nikon fluorescence microscopy. No immunoreactivity was observed in the negative controls.
Avidin-Biotin-Peroxidase Complex Reaction Revealed with DAB (DAB reaction): The slide-mounted vertical and oblique sections were removed from the freezer, kept in a heating oven at 37°C for 30 minutes, rinsed once in 0.1 M PBS (5 minutes) and pretreated by incubation in a solution of the 0.2 M boric acid (pH 9.0, 65°C) for 25 minutes. After freshening, the sections were incubated under constant agitation in a solution containing 1% hydrogen peroxide in methanol for 20 minutes. Sections were rinsed 3 times (5 minutes each) in a 0.05% PBS/Tween 20 solution and incubated with horse normal serum in PBS for 1 h. Without further rinsing, sections were then incubated in a PBS solution containing 1:500 of the commercial primary polyclonal goat antibody against avian QR2 enzyme, NQO2: N15 (sc-18574) overnight. As a negative control to check the non-specific binding of the secondary antibody, 10% horse serum, rather than the primary antibody, was used in this step. After, sections were rinsed in PBS/Tween 20 solution for 5 minutes (3 times), and incubated in a PBS solution containing the horse anti-goat biotinylated secondary antibody (1:100) for 2 h. Both primary and secondary antibodies were incubated at room temperature (20 °C). Sections were rinsed again for 5 min (3 times) in the PBS/Tween 20 solution under agitation, and incubated with the developed Avidin-Biotin-Peroxidase Complex (Vectastain ABC Kit, PK-4005) for 60 minutes. The peroxidase was visualised incubating the sections in a PB solution containing 0.05% DAB, 0.08% imidazole and 0.05% hydrogen peroxide. After the DAB reaction, sections were rinsed 3 times (3 minutes each) in 0.1 M phosphate buffer, dehydrated using alcohols and xylene, and cover slipped. Some sections were also counterstained or stained with cresyl violet for visualization of the nucleus. All sections stained with the different histological methods were surveyed by light microscopy (Nikon Eclipse 50i). No immunoreactivity was observed in the negative controls.
Data Analysis
A result was considered significant when repeated at least three times. The software Image J® was used only for slight adjusts in bright and contrast.
Results and Discussion
The Retina of the Early PH Chicks
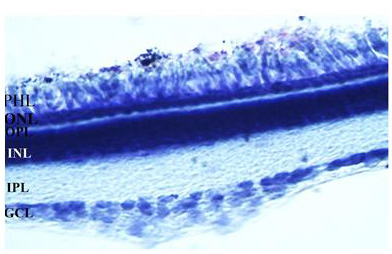
In this study, the distribution of the QR2 was investigated in the retinal layers of the early PH chick. The same development stage was used in the previous morphological and biochemical studies that showed the QR2 presence in these retinas [9,18,26]. The chick retina is avascular and its layers are well-demarcated in the early PH chicks used in this research (Figure 1).
Figure 1: The image of a 30 μm vertical retina section stained with cresyl violet is showing retinal layers of the early PH chicks. GCL: ganglion cell layer, IPL: Inner plexiform layer, INL: Inner nuclear layer, OPL: Outer plexiform layer, PHL: Photoreceptor layer. The magnification was 400xs.
The QR2 Enzyme Distribution in the Retinal Layers of the Early PH Chicks Visualized by Immunofluorescence
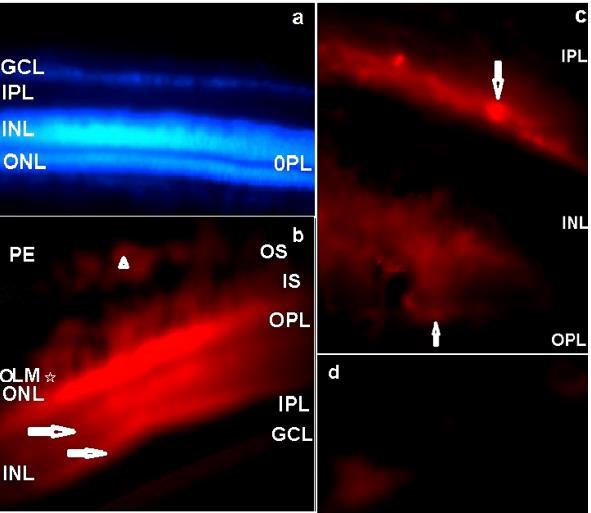
In the central retina, the QR2 enzyme was mainly localized by immunofluorescence in the Retinal Pigment Epithelium (RPE), in the Outer Nuclear Layer (ONL), in the Inner Segments (IS) and in the Outer Segments (OS) of the photoreceptors. QR2 immunoreactivity was also observed in the inner and outer borders of the Inner Nuclear Layer (INL). The ganglion cells and neural fiber layers (GCL/NFL) were also QR2 positive. On the other hand, the inner and the outer plexiform layers (IPL and OPL) were not positive to QR2 immunofluorescence in this study. Absence of the immunoreactivity was also observed in the Outer Limiting Membrane (OLM) (Figures 2a&2b). The OLM is localized between the body and the inner segment of the photoreceptors. This membrane is a semipermeable barrier with very important structural functions. A confocal microscopy analysis of the OLM is required to discard definitively the presence of the QR2 [27].
Figure 2: The distribution of the QR2 enzyme in a vertical retinal section of the early PH chick is shown by Texas Red immunofluorescence.
a) Texas Red negative control counterstained with DAPI. No Texas red immunofluorescence was observed when the blue filter was replaced by the red filter. The magnification was 400xs.
b) QR2 immunoreactivity in the PE, OS, IS, ONL and in the GCL. Arrowhead indicates QR2 immunoreactivity in a non-neural cell in the PE. Arrows point to bright linear bands localized in the outermost part and in the innermost part of the INL. The magnification was 400xs.
c) The amplified image of the areas pointed with horizontal arrows in the innermost and in the outermost parts of the INL in “b”, showing amacrine-like cells (down arrow) and horizontal-like cells (up arrow). The magnification was 1000xs.
d) QR2 immunopositive ganglion cells (magnification 1000xs). PE: pigment epithelium; OS: Outer segments of the photoreceptors; IS: Inner segments of the photoreceptors; OLM: Outer limiting membrane; ONL: Outer nuclear layer; OPL: Outer plexiform layer; INL: Inner nuclear layer; IPL: Inner plexiform layer, GCL: Ganglion cells layer.
The QR2 immunofluorescence pattern observed in this study was in accord with the cytosolic localisation of this enzyme showed by other authors [28]. In addition, the QR2 localization in bodies of the retinal cells is in agreement with the previously published results in the cerebral cortex of the human and rodent, in the hippocampus formation of the human and rodent [29], and in whole neural retinas of the early PH chick [24] and of the juvenile turtle [18]. The observed pattern of the QR2 immunofluorescence in the ONL presented herein (Figure 2b) suggests that QR2 is present in both cones and rods photoreceptors, because in chick retina cones are in superior quantity relative to rods, but rods have longer OS that touch to RPE [30], as showed herein (Figure 2b). The QR2 presence in photoreceptors is a found that links this enzyme with melatonin and dopamine synthesis because these cells are both the main place of the melatonin synthesis [15] and an alternative place of the dopamine synthesis, as showed by the presence of the DOPA decarboxylase in photoreceptors since early developmental stages in the chick retina [31]. The QR2 presence in the OS of the photoreceptors also is a link between this enzyme with melatonin and dopamine functions. This occurs because the photoreceptor strength is dependent on of the renewal of the OS, which is a melatonin effect, and of the phagocytosis of these OS by the RPE cells, which is an effect mediated by dopamine [32]. As the presence of the QR2 mRNA in the human pigment retinal cells in culture was previously showed by other authors [16], it is possible to suppose that the presence of the QR2 in the non-neural retina has evolutionary importance.
In general, bipolar-photoreceptors synapses are modulated by horizontal neurons located in the outmost part of the retinal INL, while bipolar-ganglion cells synapses are modulated by amacrine cells located in the innermost part of the INL, which encloses bodies of the bipolar, horizontal, amacrine and Muller glia cells. The characterization of these retinal cells can be done by their proper morphology and positioning in the retinal layers [33], furthermore the width of the INL is progressively smaller from central to peripheral retina [34]. In this study, highly immunoreactive parallel linear bands were respectively located in the outmost part and in the innermost part of the INL of the central retina, but they were not localized in the periphery of the retina (data not shown). These bright areas were also parallel to another linear band highly immunoreactive in photoreceptor layer, nearby the OPL (Figure 2b). Higher magnification of the linear bright bands located along of the outermost and of the innermost borders of the INL showed respectively horizontal-like cells and rounded and elongated amacrine-like cells (Figure 2c). These clustered QR2-positive cells are related to modulation of the synapses in the central retina, but it is not possible to infer the role of the QR2 in this because the type of the amacrine and horizontal cells were not characterized.
The dopamine synthesis and the dopamine selective transporter are located in amacrine cells [7]. Additionally, a recent study in the mammalian retina shows that the dopamine uptake and re-uptake also occur by non-selective transporters located in ganglion cells, in horizontal cells, in photoreceptors and in retinal pigment epithelial cells [26]. A more advanced investigation using double-staining immunofluorescence protocols is necessary to show the type of the amacrine-like cells that were QR2 positive in chick retina, and the presence of the non-selective dopamine transporters in the QR2 positive retinal cells shown herein. Ganglion cells presenting QR2 immunoreactivity were also observed by immunofluorescence in this study (Figure 2d). Regarding the characterization of the ganglion cells positive to QR2 as melatoninergic cells, it is another interesting point for further investigation because some ganglion cells have melatonin synthesis machinery in chick retina [35].
QR2 Enzyme Distribution in Retina Sections of the Early PH Chick Visualized by DAB Reaction
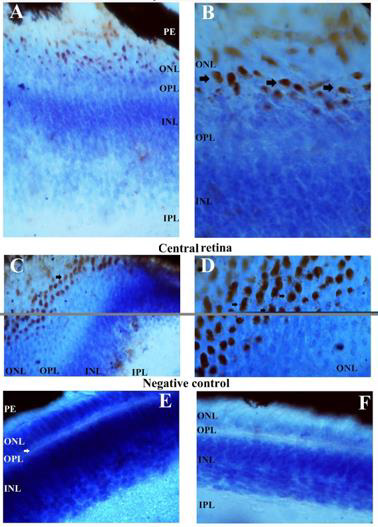
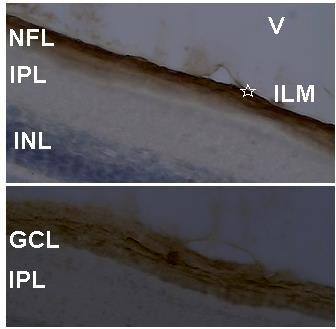
The QR2 enzyme distribution was previously investigated by DAB reaction in vertical and oblique retinal sections of the early PH chick. The results found in the vertical sections match well with those obtained using Texas Red immunofluorescence. The exception was the presence of the QR2 immunosignal in amacrine- and in horizontal- like cells only in sections assayed by immunofluorescence. This difference occurred either due to the retinal sections analysed or due to the DAB protocol utilized in this study. The main results obtained using DAB in vertical sections were the QR2 distribution in the RPE, in the photoreceptors layer, in the GCL and in the NFL (data not shown). Oblique sections were used to better visualize the QR2 presence in the non-neural retina, in the ONL, in the GCL, in the NFL and in the Inner Limiting Membrane (ILM) ( Figure 4). The distribution of the QR2-positive photoreceptors was higher in the central than in the peripheral areas of the retina (Figure 3). This distribution was in accord with the expected for the density of these cells in each area investigated [34].
The presence of the QR2 in the photoreceptor (Figure 3) is important to be highlighted because they are the second place of the melatonin synthesis in both avian retina [36]and mammalian retina [37], losing only to the melatonin production in the pineal gland [12]. Thus, the melatonin concentration into citosol of the QR2-positive photoreceptors must reach suitable levels to modulate the QR2 activity as in mammalian as in avian retinas. However, the DOPA-decarboxylase is present in photoreceptors in the chick [32], while in the mammalian photoreceptors never was noticed the presence of this enzyme. The NFL and ILM were also QR2 positive in the central retina and in the peripheral retina (Figure 4). The QR2 immunoreactivity in the area corresponding to ILM was visualized only in oblique sections in this study (Figure 4). The ILM is a basement membrane attached to the Muller cells footplates on the inner surface of the retina and to the cortical vitreous gel on the other side. It is composed of the structural macromolecules, having mainly structural functions [38]. Therefore, it is unexpected the presence of the QR2 in this region. Thus, this result will be subject of the future investigation.
Figure 3: QR2 localization in the most external part of the early PH chick retina, as it appears in an oblique section staining by DAB and counterstained with cresyl violet. Immunoreactivity in the bodies of the photoreceptors is present in both peripheral and central retina, as explained in the figure. The magnification was 400xs in the images A and C, while in the images B and D the magnification was 1000xs. Images E and F are the negative controls of the secondary antibody, in the central and peripheral regions of the retina (magnification 400xs). PE: retinal pigment epithelium; OS: outer segments of the photoreceptors; ONL: outer nuclear layer; OPL: outer plexiform layer; INL: inner nuclear layer; IPL: inner plexiform layer.
Figure 4: QR2 localization in an oblique section of the most internal part of the early PH chick retina. Sections were stained by DAB and counterstained with cresyl violet. Above, the QR2 presence in the NFL and in the ILM (star) is showed. Below, the QR2 presence in a ganglion cell (middle of the figure) and in neural fibers at GCL is showed. The magnification was 400xs. V: vitreous; ILM: inner limiting membrane; NFL: neural fiber layer; GCL: ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer.
Immunoreactivity for QR2 was not showed to occur in the thinner OLM (Figures 2b, 3b&3d). This point is highlighted because the OLM is the place of the traffic of the substances between the retina and non-neural ocular tissues, functioning as a semipermeable barrier that appear to be communicating with the main retinal barriers located in the RPE [27]. The present results showing QR2 presence in the RPE are in agreement with QR2 functioning as a detoxification enzyme in the retina [39,40].
Conclusion
We conclude that QR2 has a specific distribution in retinal layers. The observed distribution in photoreceptors suggests that melatonin can be a natural QR2 modulator in the retina. As well, the canonical QR2 detoxification activity must be occurring in this tissue by the presence of this enzyme in RPE. On the other hand, QR2-positive horizontal, amacrine and ganglion cells still were not characterized regarding dopamine containing, and this should be important to reinforce the previous pharmacological results showing dopamine quinone as a natural QR2 cosubstrate.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.