Neuroprotective Effects of a Cameroonian Drink, Tenghõ, on Memory Impairment and Neuroinflammation Induced by Scopolamine on Ovariectomized Wistar Rat
Introduction
Neurodegenerative diseases are defined as hereditary and sporadic conditions, which are characterized by progressive nervous system dysfunction. They include diseases such as Alzheimer’s Disease (AD) and other dementias, Brain Cancer, Epilepsy, Stroke, Parkinson’s Disease, Multiple Sclerosis, Huntington’s Disease, and others. Alzheimer’s disease is the most common form of dementia and is characterized by a progressive decline in cognitive function Farooqui [1]. Alzheimer’s Disease damages the cerebral cortex, a vulnerable brain region implicated in memory, emotion, cognition, and decision-making behavior and is characterized by progressive neuronal loss, but the mechanisms of cell death at different stages of the disease remain unknown Darya, et al. [2]. Care and support of patients with dementia has wide-ranging consequences for families, health-care systems, and society as a whole Etters, et al. [3]. The vast majority of women experience spontaneous cessation of menstruation between the ages of 47 and 55 and the physiological changes associated with menopause cause uncomfortable symptoms in some women. Most importantly, estrogen deprivation resulting from menopause, in combination with age-related factors, disproportionately increases the risk of many neurodegenerative diseases, such as AD Yaffe, et al. [4].
In women, circulating ovarian hormones not only play a main role in reproductive behavior and sexual differentiation, they also contribute to emotion, memory, neuronal survival and the perception of somatosensory stimuli Gasbarri, et al. [5,6]. Ovarian hormones have also been suggested to regulate affective disorders and learning memory beyond their role in pain modulation Gasbarri, et al. [5]. In experimental animals, ovariectomized is a common method to deplete animals of their gonadal hormones. In females, the absence of the ovaries induces a drastic decrease of circulating estrogens Zhao, et al. [7]. Inflammation is increasingly recognized as a causal factor in the pathology and chronic nature of central nervous system (CNS) disease Block, et al. [8]; while diverse environmental factors have been implicated in neuroinflammation leading to CNS pathology Craig, et al. [9]. For that, a combinatorial cocktail approach is suggested as a rationale intervention to attenuate chronic inflammation and confer neuroprotection in Alzheimer’s disease (AD) Mc Larnon [10]. The requirement for an assemblage of pharmacological compounds follows from the host of pro-inflammatory pathways and mechanisms present in activated microglia in the disease process Mc Larnon [10]. In this study, we used the Tenghõ’s drink as a combinatorial approach to relieve memory disorders.
Tenghõ is a drink made by several Cameroonian spices including Zingiber officinale, Allium sativum, Cymbopogon citratus, Ocimum basilicum, Petroselinum crispum, and Bee propolis. In Cameroon, the drink is empirically used for the treatment of inflammation, pain and anxiety. In our previous studies (not yet published), Tenghõ shows an anti-inflammatory and analgesic activities. Knowing the link between inflammation, oxidative stress and neurodegenerative disorders, the present study aimed to assess neuroprotective effects of Tenghõ on memory impairment and neuroinflammation induce by scopolamine on ovariectomized Wistar rat.
Material and Methods
Plant Collection and Preparation of Tenghõ’s Drink
The ingredients used for the preparation of Tenghõ were collected in Dschang (West Region of Cameroon), and consist of garlic (Allium sarivum), basil (Ocimum basilicum), parsley (Petroselinum crispum), lemon grass (Cymbopogon citratus) and propolis. The mixture of these ingredients was macerated in 40% ethanol. The filtrate obtained after filtration with a Wattman paper No4 was lyophilized to obtain a crude dry extract. The therapeutic dose of 400 mg/kg was extrapolated from the traditional used in humans and surrounded by the doses of 200 and 600 mg/kg.
Experimental Animals
Adult female Wistar rats (7-8 weeks old, 115-150 g) were used for the test. Healthy animals were bred in the animal house of the Department of Animal Biology and Physiology, University of Yaounde I. They were maintained under normal laboratory condition of temperature (25 ± 2 °C) with a natural ~12 h light/ dark cycle. Animals had free access to tap water and soy free rat chow ad libitum. Animal handling and experiments were carried out in conformity with the European Union on Animal Care (CEE Council 86/609; Reg.no.FWA-IRD0001954) guidelines adopted by the Institutional Ethics Committee of the Cameroon Ministry of Scientific Research and Technology Innovation.
Chemicals
Scopolamin (SCOPO) were obtained from Jiangsu Huayang pharmaceutical Co. Ltd Zhiongxing (China) and was used to induce neuroinflammation. Piracetam (Nootropyl ®, PIR) was purchased from UCB SA, Braine-l’Alleud-(Belgium) and was used as reference drug.
Behavioral Assessments
Y Maze Task Test: The Y-maze test is a recognition memory test used to assess short-term spatial working memory. It is a test sensitive to hippocampal damage, genetic manipulation and amnesic drugs. The Y-maze test is particularly useful as an initial test of memory function in rodents Kraeuter, et al. [11]. The device used was made of wood, and consisted of three arms (11 x 50 x 32 cm each) separated between them by an angle of 120°. The block allowing to close one arm of the device, was placed at 5 cm from the center of the device. In this study, the protocol described by Kraeuter, et al. [11] was used. All arm entries were noted sequentially so that the total number of arm entries, as well as the sequence of entries, was recorded. The time spend in the novel arm was recorded and percentage of good First arm entered was calculated according to de formula:
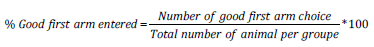
Morris Water Maze Test: The Morris Water Maze (MWM) test is widely used to study spatial and long-term memory Morris [12,13]. The water maze used in this study was consists of a black round tank, which has a diameter of 150 cm and height of 54 cm and is filled with water to a depth of 38 cm. The submerged platform (9.0 cm in diameter, 1.0 cm below the water surface) is invisible in the target quadrant. Fixed, extra-maze visual cues were present at various locations around the maze (posters) in the room with constant brightness (25 lux). In this study, the protocol described by Vorhees and Williams was used Vorhees [14]. In the training session days, the latency time of entry in the target quadrant was recorded and at the day of test session (day 5), the latency time of entry, the number entry and the time spent in the target quadrant were recorded. All parameters were recorded by a video tracking system connected to a computer directly above the water tank. The percentage of Time spent in the target quadrant was calculated according to the formula:
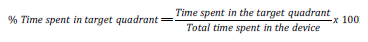
Experimental Design: Twenty-five females Wistar rat were ovariectomized and divided in 5 groups of 5 animal each: Negative control (OVX) and Positive control (PIRA) receiving distilled water, three tests groups (TEN 200, TEN 400 and TEN 600) receiving the Tengho’s drink at the doses of 200, 400 and 600 mg/kg respectively. Five others animals were used as Sham (Control group) and treated with distilled water. After 28 days of estrogen decline, all animal received an injection of Scopolamine (0.6 mg/kg, i.p.) during 14 days, excepted the sham group. The drink was administered orally from day 28 post ovariectomy to day 51. At the days 38 and 46 post ovariectomized, animals were suggested to the Y maze and Morris water maze tests respectively. Animals were sacrificed after 52 days post ovariectomy and brains were harvested. The right cerebral hemisphere was ground in Tris buffer for evaluation of oxidative stress parameters (MDA, SOD and nitrites levels and Catalase activity) in homogenate, while the right cerebral hemisphere was fixed in 10% formalin for H&E and cresyl violet staining as well as immunohistochemistry (Iba-1 and GFAP for microglia and astrocytes expression respectively).
Biochemical Assay
The levels of malondialdehyde (MDA), superoxide dismutase (SOD) and Nitrites as well as Catalase activity were evaluated according to the protocols described by Wilbur, et al. [15-17] respectively
Sectioning for Histology and Immuno-Histochemistry
Sections of 5-μm thickness were obtained from brain tissues embedded in paraffin wax using a standard microtome.
Haematoxylin & Eosin and Cresyl Violet Staining: Brain tissue sections were processed for and Hematoxylin & Eosin and cresyl violet staining based on the work of Dawson, et al. [18,19]. Sections were deparaffinized in 2 changes of xylene and thoroughly dehydrated in solutions of graded alcohol concentration (i.e. 100%, 90%, 80% and 70%). Sections were thoroughly rinsed in 2 changes of distilled water and stained. Immediately after staining, sections were dehydrated through descending alcohol grades (80%, 90%, and 100%), cleared in 2 changes of xylene, and subsequently mounted wet in dibutyl phthalate xylene (DPX) a synthetic mountant, with the use of coverslips. The slides were allowed to dry and prepared ready for microscopy.
Immunohistochemically Labeling with Antibodies: Immunostaining was done in accordance with the standard protocol described by Folarin, et al. [19]. Paraffin sections were dewaxed, rehydrated and immersed in distilled water. Antigen retrieval was done in 10 mM citrate buffer (pH = 6.0) for 25 min, with subsequent peroxidase quenching in 3% H2O2/methanol. All sections were blocked in 2% milk for 1 h to avoid non-specific background staining. Brain sections were immuno-labelled with the following antibodies: anti-Iba-1 (1:1000; Abcam, Cambridge, MA, USA) and anti-GFAP (1:1000; Dako, Denmark). Each antibody was diluted in 1% PBS milk and 0.1% Triton X detergent (to facilitate quick penetration of antibody) and incubated over night at 4oC. HRP-conjugated secondary antibodies in VECTA-STAIN kit (Vector Labs, Burlingame, USA) was subsequently used to detect the bound antibody based on the manufacturer’s protocol. The end product of reaction was improved using 3, 3′-diaminobenzidine as a chromogen (DAB) (1:25 dilution) for 5 min. Sections were thoroughly dehydrated in solutions of graded alcohol concentrations, then passed through xylene, and mounted wet in DPX, cover slipped and allowed to dry. With identical light intensity and exposure settings, images were taken with a bright fild microscope (Biomicroscope, YJ-2005 series) equipped with × 4 × 10 and × 40 dry and × 100 oil objectives and a cameral Inspiration Marvotech (MYCH-10 L) and AmScope ToupView 3.2 software’s connected to a monitor screen of a laptop computer.
Statistical Analysis
All data were expressed as the mean ± standard error of the mean (S.E.M) and analysed with one-way ANOVA followed by the Dunnett post-test (GraphPad Prism® Software, version 5.03, San Diego, CA, USA). The p values < 0.05 were considered significant.
Results
Neuroprotective Effects of Tenghõ’s Drink on Memory Impairment Induced by Scopolamin on Ovariectomized Wistar Rat
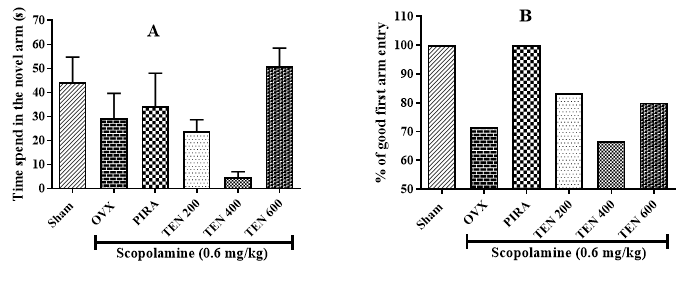
Effects of Tenghõ’s Drink on Spatial and Short-Term Memory Evaluate by the Y-Maze Test: The analysis of results presented on (Figure 1) showed that compared to the Sham group, Scopolamine injection induced a non-significant decrease of the time spent in the novel arm (Figure 1A) and the percentage of good first arm entry (Figure 1B). In comparison to the negative control group, the treatment with the Tenghõ’s drink at the dose of 600 mg/ kg as well as Piracetam induced a non-significant increase of the time spent in the novel arm (Figure 1A), while the Tenghõ’s drink at the dose of 200 and 600 mg/kg induced a non-significant increase of the percentage of good first arm entry (Figure 1B). Data are expressed as mean ± S.E.M., n = 5 per group. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals, which received Scopolamine and distilled water; PIRA: Ovariectomized animals, which received Scopolamine and Piracetam 300 mg/kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received Scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively.
Figure 1:
A. Effects of Tenghõ’s drink on the time spent in the novel arm and
B. The percentage of good first arm entry in the Y maze.
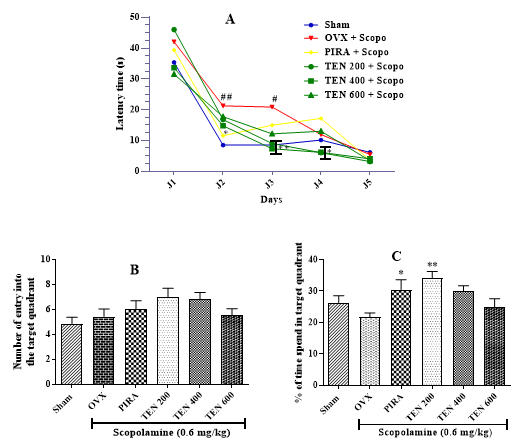
Effects of Tenghõ’s Drink on Spatial and Long-Term Memory Evaluate by the Morris Water Maze Test: The analysis of the latency time of entry into the target quadrant in the training session of the MWM test shows that compared to the Sham group, ovariectomized animal that received only Scopolamine showed an increase of this parameter (Figure 2A). This increase was significant (p < 0.05; p < 0.01) in day 2 and 3. Animals treated with Tenghõ, as well as Piracetam, showed a decreased latency time of entry into target quadrant compared to negative control group (Figure 2A). This effect was significant in day 3 (p < 0.01) and day 4 (p < 0.05) with the drink at the doses of 200 and 400 mg/kg (Figure 2A). In the day of test session (day 5), compared to the negative control group, the treatment with Tenghõ’ drink induced a non-significant decrease of the latency time of entry into the target quadrant. (Figure 2B) presented the effect of Tenghõ’ drink in the number of entry into the target quadrant at the day of test session (day 5). No significant difference was observed between Sham and OVX groups. Compared to the negative control group, Tenghõ at doses of 200 and 400 mg/kg as well as Piracetam, induced a non-significant increase in the number of entry into the target quadrant. At the day of test session (day 5), compared to the negative control group, ovariectomized animal treated with Scopolamine only induced a non-significant reduction of the percentage of time spent in the target quadrant (Figure 2C).
The administration of Tenghõ at doses of 200 and 400 mg/kg as well as Piracetam, induced an increase of the percentage of time spent in the target quadrant in comparison to the negative control group. The effect was significant (p < 0.01) with the drink at the dose of 200 mg/kg (Figure 2C). Data are expressed as mean ± S.E.M., n = 5 per group. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals, which received scopolamine and distilled water; PIRA: Ovariectomized animals, which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. #p < 0.05, ##p < 0.01 vs. Sham; *p < 0.05, **p < 0.01 vs. OVX (one-way Anova followed by Dunnett’s post test).
Figure 2: Effects of Tenghõ’s drink on latency time of entry into (A), the number of entry into (B) and the percentage of time spent (C) in the target quadrant of the Morris water maze.
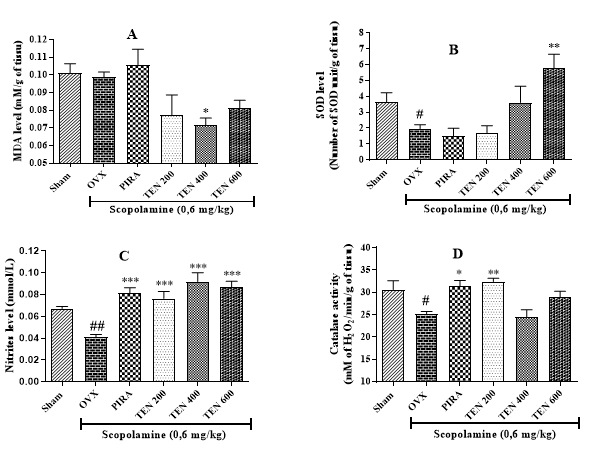
Effect of the Tenghõ’s Drink on Some Oxidative Stress Parameters: The analysis of (Figure 3) shows that compared to Sham group, animals of negative control group presented a nonsignificant change in the MDA level (Figure 3A) and a significant (p < 0.01; p < 0.05) decrease of the of SOD and Nitrite levels and the Catalase activity Figure 3A, B and C respectively). The treatment of ovariectomized rat with Tenghõ’s drink induced a significant (p < 0.05) decrease of the MDA level (dose 400 mg/kg, (Figure 3A), and a significant (p < 0.01) increase of the of SOD (dose 600 mg/ kg, (Figure 3B) and Nitrite (all tested dose, (Figure 3C) levels and the Catalase activity (dose 200 mg/kg, (Figure 3D). Data are expressed as mean ± S.E.M., n = 5 per group. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals, which received scopolamine and distilled water; PIRA: Ovariectomized animals, which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. #p < 0.05, ##p< 0.01 vs. Sham; *p < 0.05, **p< 0.01, ***p< 0.001 vs. OVX (one-way Anova followed by Dunnett’s test).
Figure 3: Effects of Tenghõ’s drink on the MDA (A), SOD (B) and Nitrite (C) levels and the Catalase activity (D).
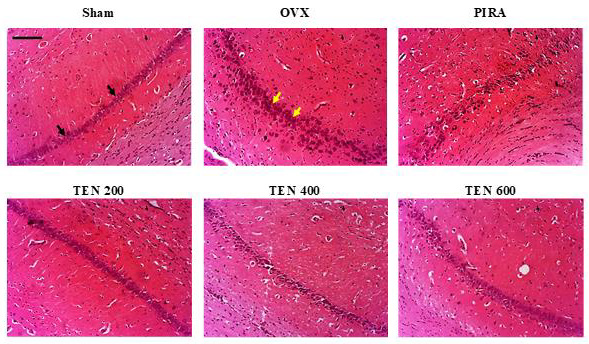
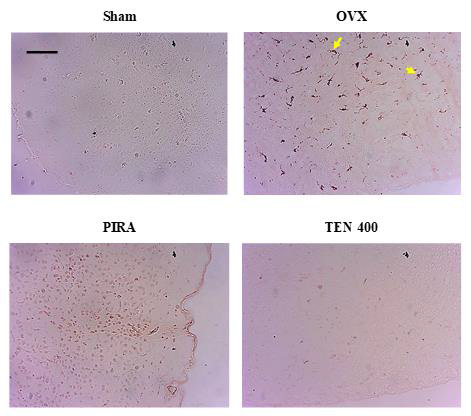
Figure 4: Effects of Tenghõ’s drink on microarchitecture (H&E staining) of CA1 region of the hippocampus.
Effects of Tenghõ’s Drink on the Microarchitecture of the Hippocampus: (Figures 4 & 5) shows the H&E and cresyl violet stain respectively of the hippocampal cells (CA1 region). Compared to Sham group, Scopolamine induced neurodegeneration in animals of negative control group. We noted an increase in the necrotic cells (yellow arrow) in the CA1 regions of the hippocampus of OVX group (Figures 4 & 5). The treatment with Tenghõ’s drink, as well as Piracetam reversed the neurodegenerative effect of Scopolamine in the CA1 region of the hippocampus. OVX group presented more necrotic cells (yellow arrow) than TEN 200, TEN 400 and TEN 600 groups (Figures 4 & 5). Photomicrographs of H&E stain of CA1 region of the hippocampus, X200, scale bar, 20 μm. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals, which received scopolamine and distilled water; PIRA: Ovariectomized animals which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. Black arrow = normal cells and yellow arrow = necrotic cells.
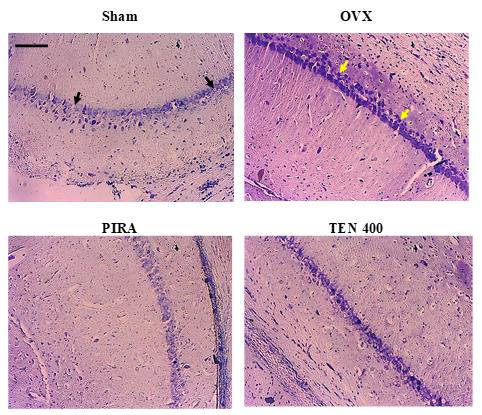
Figure 5: Effects of Tenghõ’s drink on microarchitecture (cresyl violet staining) of CA1 region of the hippocampus.
Photomicrographs of cresyl violet stain of CA1 region of the hippocampus, X100, scale bar, 20 μm. Sham: Animals having undergone white surgery which received distilled water; OVX: Ovariectomized animals which received scopolamine and distilled water; PIRA: Ovariectomized animals which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. Black arrow = normal cells and yellow arrow = necrotic cells.
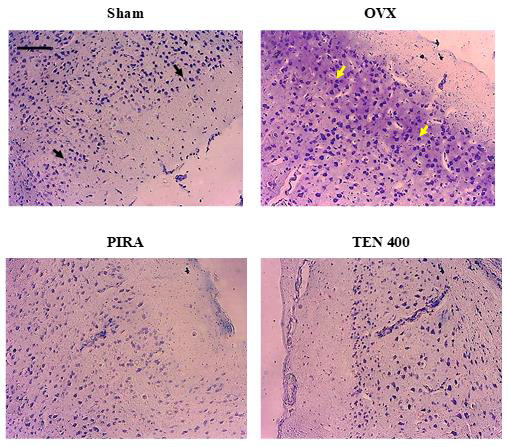
Effects of Tenghõ’s Drink on the Microarchitecture of the Cingulate Cortex: As shown on (Figure 6), Scopolamine induced neurodegeneration in animals of OVX group in comparison to Sham group. We noted an increase in the necrotic cells (yellow arraw) in the cingulate cortex of OVX group (Figure 6). The treatment with Tenghõ’s drink, as well as PIRA reversed the neurodegenerative effect of Scopolamine in the cingulate cortex. Animals of OVX group presented more necrotic cells (yellow arrow) than those treated with Tenghõ’s drink (Figure 6). Photomicrographs of cresyl violet stain of cingulate cortex, X100, scale bar, 20 μm. Sham: Animals having undergone white surgery which received distilled water; OVX: Ovariectomized animals which received scopolamine and distilled water; PIRA: Ovariectomized animals which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. Black arrow = normal cells and yellow arrow = necrotic cells.
Figure 6: Effects of Tenghõ’s drink on microarchitecture (cresyl violet staining) of cingulate cortex.
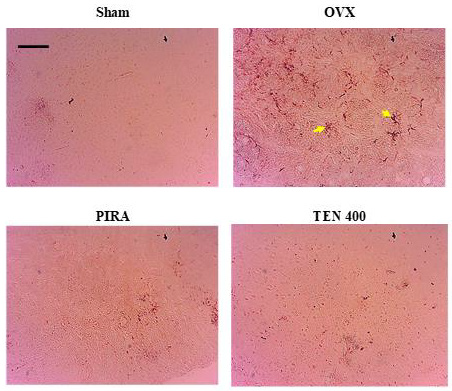
Effects of Tenghõ’s Drink on Ionized Calcium-Binding Adapter Molecule 1 And Glial Fibrillary Acidic Protein Expression in the Cingulate Cortex: Compared to Sham group, the administration of Scopolamine in ovariectomized rat resulted in an increase in Iba-1 and GFAP-positive microglia and astrocytes respectively (yellow arrow) in the cingulate cortex (Figures 7 & 8). The animals treated with Tenghõ’s drink showed less Iba-1 and GFAP expression of activated microglia and astrocytes in the cingulate cortex (Figures 7 & 8). Microglia activation (yellow arrow) as shown by amoebic isoforms was more in OVX group than groups of animals treated with the Tenghõ’s drink (Figure 7). Astrogliosis (yellow arrow) was greater in OVX group than groups of animals treated with the Tenghõ’s drink (Figure 8). Photomicrographs of Iba-1-immunolabelled microglia in cingulate cortex, X100, scale bar, 20 μm. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals which received scopolamine and distilled water; PIRA: Ovariectomized animals which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. Yellow arrow = activated microglia.
Photomicrographs of GFAP-immunolabelled astrocyte in cingulate cortex, X100, scale bar, 20 μm. Sham: Animals having undergone white surgery, which received distilled water; OVX: Ovariectomized animals which received scopolamine and distilled water; PIRA: Ovariectomized animals which received scopolamine and Piracetam 300 mg / kg; TEN 200, TEN 400 and TEN 600: Ovariectomized animals, which received scopolamine and Tenghõ’ drink at the doses of 200, 400 and 600 mg/kg respectively. Yellow arrow = activated astrocytes.
Discussion
The hypothesis of specific neuroanatomical and neurophysiological effects of estrogen on the brain may explain the correlation between estrogen deficiency and cognitive disorders such as dementia of the Alzheimer’s Disease (AD) Genazzani, et al. [20]. To accentuate the effects of this model of neurocognitive disorders, the administration of Scopolamine was associated to ovariectomy. Thus, the present study aimed to assess neuroprotective effects of Tenghõ’s drink on memory impairment and neuroinflammation induce by Scopolamine on ovariectomized Wistar rat. Neuroprotective effects of the Tenghõ’s drink on spatial, short-term and long-term memory were evaluated using Y-Maze and the Morris Water Maze test respectively. The obtained results on the assessment of short-term memory showed that the injection of Scopolamine at the dose of 0.6 mg/kg for 14 days in ovariectomized rats induced a decrease of the time spent in the novel arm and the percentage of good first arm entry compared to the normal control. These effects marked a slight impairment of spatial and short-term memory compared to the normal control (Sham). Indeed, estrogen deprivation resulting from menopause or ovariectomy causes neurocognitive impairment as early as 2 weeks post-ovariectomy Djiogue, et al. [21]. In addition, Scopolamine creates an animal model for AD by disrupting the cholinergic system and altering learning and memory processes Kim, et al. [22]. Scopolamine would increase acetylcholine activity and decrease performance in all memory tasks, confirming the occurrence of cognitive impairment in the animals Xiao, et al. [23].
However, the treatment of ovariectomized rat, receiving Scopolamine, with the Tenghõ’s drink as well as Piracetam induced an increase of the time spent in the novel arm at the dose of 600 mg/ kg. The drink also induced an increase of the percentage of good first arm entry at the dose of 200 and 600 mg/kg. This suggests that Tenghõ’s drink would improve the spatial and short-term memory of animals and could be endowed with secondary metabolite able to reversed the adverse effects induced by estrogen depletion and Scopolamine on memory. These secondary metabolites could act as Piracetam on the central nervous system to improving learning, memory and brain metabolism Katarzyna, et al. [24] by facilitating cholinergic and excitatory amine neurotransmission Vernon [25]. The Morris Water Maze is a commonly used experimental method to assess spatial learning and memory in animal models Hosseini, et al. [26]. Using the Morris water maze test, we found that injection of Scopolamine (0.6 mg/kg) in ovariectomized rats impaired spatial and long-term memory. Scopolamine induce a significant increase of latency time of entry into the target quadrant in the training session (day 1 to 4) as well as a reduction of the percentage of time spent in the target quadrant (day 5) compared to the normal control. Indeed, an earlier study by Markowska [27] reported that after ovariectomy, cognitive impairment was progressive.
Estrogen deprivation is likely to initiate or worsen degenerative changes caused by oxidative stress and reduce the brain’s ability to maintain synaptic connectivity and cholinergic integrity, leading to cognitive decline in the elderly and in people with diseases Gandy, et al. [28]. In addition, Scopolamine causes inhibition of cholinergic signaling Vogel [29]. In contrast, compared to OVX group, the treatment with Tenghõ’s drink improved spatial memory in the Morris water maze. This was marked by a significant reduction of latency time of entry into the target quadrant at the doses of 200 and 400 mg/kg, and a significant increase of the percentage of time spent in the target quadrant at the dose of 200 mg/kg. Several plant-derived compounds show anticholinesterase activity in preclinical studies Konrath, et al. [30] and may thereby improve cognitive decline. Indeed, these neuroprotective effects could be due to the compounds contained in the drink. For example, Garlic found Allium sativum, has been studied in the prevention of AD. Its administration in laboratory animals appeared to be able to improve memory and cognitive functions and increase serotonin activity Mathew [31,32]. Similarly, luteolin (flavonoid) and Apigenin (flavone) found in parsley (Petroselinum crispum) has shown a clear neuroprotective effect by inducing preventive activity on neuroinflammation and improved memory and learning abilities Mecocci, et al. [33-35].
Oxidative stress is one of the main factors involved in the pathogenesis of neurodegenerative disorders, including AD Liu, et al. [36]. Compared to Sham group, injection of Scopolamine 0.6 mg/kg for 14 days in ovariectomized rats induce a significant decrease of the SOD and Nitrite levels and the Catalase activity. No significant change in MDA level was observed. Scopolamine treatment has been associated with elevated oxidative stress Jang, et al. [37]; it induces a decrease of the brain activity of antioxidant enzymes Abhinav, et al. [38]. In addition, excessive oxidative stress can lead to cell death (apoptosis) Floyd, et al. [39]. The treatment of ovariectomized rat with Tenghõ’s drink as well as Piracetam induced a significant decrease of the MDA level at the dose of 400 mg/kg, and a significant increase of the of SOD level at the dose of 600 mg/kg, a significant increase of Nitrite level at all tested dose and a significant increase of the Catalase activity in comparison to OVX group. These effects suggested that the drink is endowed with antioxidant properties. Indeed, many plants, including vegetables and fruits, are natural antioxidant bases that can protected again oxidative stress and play a key role in chemoprevention of diseases that have their etiology and pathophysiology in reactive oxygen species (ROS) Kavya, et al. [40]. Some compounds contained in the Tenghõ drink, such as garlic (allium sativum) are believed to have antioxidant effects.
Garlic exerts its antioxidant effect by scavenging ROS, enhancing activity of antioxidant enzymes and increasing glutathione levels in cells Santhosha, et al. [41]. These positive effects can also be attributed to antioxidants such as flavonoids Rohini, et al. [42] contained in the drink. Brain histology, using H&E and cresyl violet stain, was performed to observe changes in the microarchitecture of hippocampus and cerebral cortex. Compared to Sham group, Scopolamine induced neurodegeneration in animals of negative control group (OVX). This was marked by increase of the necrotic cells in the CA1 regions of the hippocampus and cingulate cortex of the animals of OVX group. It is known that scopolamine induces apoptosis of nerve cells Jahanshahi, et al. [43]. Thus, changes in the rat hippocampus as well as cortex would induce changes in synaptic transmission, resulting in relevant changes in cognitive functions Knafo, et al. [44]. Compared to OVX group, the treatment with Tenghõ’s drink, as well as Piracetam reversed the neurodegenerative effect induced by Scopolamine in the CA1 region of the hippocampus and cingulate cortex. These results suggested that the drink could has neuroprotective effect in hippocampus and cerebral cortex.
The neurodegeneration observed with histological analysis was confirm by immunohistochemistry analysis of Iba-1 and GFAP for microglia and astrocytes expression respectively. It is well known that microglia activation and astrogliosis are characteristic features of CNS lesions and recruitment of microglia is reportedly regulated by astrocytes Gudi, et al. [45]. Compared to Sham group, the administration of Scopolamine in ovariectomized rat resulted in an increase in Iba-1 and GFAP-positive microglia and astrocytes respectively in the cingulate cortex. These results suggested that the administration of Scopolamine for 14 days in ovariectomized rats induced neuroinflammation. Microglial activation is one of the earliest events in AD pathology resulting in an increased of pro-inflammatory effects and the main cause of neurotoxicity Heneka, et al. [46]. Alzheimer’s Disease is associated with specific damage due to astrocytes, which may occur in the early stages of the disease and contribute to cognitive abnormalities Verkhratsky, et al. [47]. The animals treated with Tenghõ’s drink showed less Iba-1 and GFAP expression of activated microglia and astrocytes in the cingulate cortex. Microglia activation as shown by amoebic isoforms was more expressed in OVX group than groups of animals treated with the Tenghõ’s drink. Astrogliosis was greater in OVX group than groups of animals treated with the Tenghõ’s drink.
These results suggested that the drink could induce an inhibition of neuroinflammation causing by estrogen depletion and Scopolamine administration. The effects of the drink could be due to flavonoids compound found in some plant in the drink. Indeed, flavonoids could modulate neuroplasticity in terms of neurogenesis and synaptogenesis Spencer, et al. [48-49]. Flavonoids could also interact with neuronal and glial cell signaling pathways Moosavi, et al. [50] to perform several functions: promoting peripheral and loco regional vasodilation modulating cerebral blood flow Spencer, et al. [48-49], exert antioxidant and anti-inflammatory activity in biological systems that mitigate neuronal and endothelial damage González, et al. [51,52], act as hormonal mimetic that induce possible beneficial neurodegenerative changes (Kridawati et al., 2016).
Conclusion
The aim of the present study was to assess neuroprotective effects of Tenghõ on memory impairment and neuroinflammation induce by Scopolamine on ovariectomized Wistar rat. The results obtained showed that the Tenghõ’ drink improve short-term and long-term memory evaluate by Y maze and Morris water Maze tests respectively. The drink protected the brain against oxidative stress, microglia activation and astrogliosis in the hippocampus and cerebral cortex. Taken altogether, these results could justify the empirical use of Tenghõ’ drink for the treatment of inflammation. This drink could be considered as a functional food.



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.