Growing Teratoma Syndrome, A Rare Case
Introduction
Germ cell tumors are widely considered to be morphologically and immunophenotypically homologous to germ neoplasms arising in the gonads and extra-gonads. Classification of germ cell tumors based on the World Health Organization (WHO) was divided into germinoma (resembling seminoma of the testis and dysgerminomas the ovaries), teratoma (mature, immature, and secondary malignancies), tumor yolk sac, embryonal carcinoma, choriocarcinoma, and tumor mix [1]. Germ cell malignancies account for 10% of all non-epithelial malignancies of the ovary. The most common types of germinal malignancies include dysgerminoma and immature teratoma. 99% of teratoma in ovary was benign and the others was malignant. Histological analysis of immature teratomas showed that they consist not only of an immature component but also a mature component, the latter containing most of the immature neural tissue. Immature teratomas are classified into three grades due to the relative amount of immature neural tissue. Treatment were surgery and chemotherapy. This malignancy has a fairly good cure rate among ovarian malignancies [2].
Growing teratoma syndrome (GTS) is a rare complication between patients with germinal ovarian cell tumors. The incidence of growing teratoma syndrome occurs in 1.9-7.4% in all immature teratomas, characterized by an enlarged metastatic mass during adequate systemic chemotherapy and normal serum markers. Retro-peritoneal residual mass is a common finding after chemotherapy. It may contain mature teratomas, fibrotic tissue, or tumors. Mature teratomas, which unresponse to chemotherapy, may result from the evolution of malignant lesions after chemotherapy or may represent metastases from mature teratoma foci [3].
Case Illustration
An 18-year-old woman from Acehnese ethnicity, unmarried, came for routine check-ups to the Gynecological Oncology Polyclinic at Dr. Zainoel Abidin Hospital, Banda Aceh with complaint of abdominal pain that was felt occasionally. Other complaints such as bleeding from vaginal, nausea and vomiting were denied. The urination and defecation were still within normal limits. History of menarche at the age of 12 years, regular menstruation, 7 days of menstruation, changing sanitary napkins 3-4 times a day (before illness), and denied dysmenorrhea. Currently, the patient has no complaints during menstruation. The patient has undergone unilateral salpingo-oophorectomy due to ovarian tumor indications in 2019 with histopathological results of immature teratoma. After that, the patient underwent chemotherapy with a regimen of bleomycin, etoposide and cisplatin for 6 cycles in the same year. After chemotherapy, patients routinely underwent ultrasound evaluation and tumor markers. In the evaluation, there were no abnormalities in the ultrasound findings and tumor markers.
At this time, the patient claimed to feel a lump in the lower abdomen. Examination of vital signs were within normal limits. On physical examination found a flexible abdomen, intestinal peristalsis was found within normal limits and did not increase a palpable mass at the level of the symphysis pubis, mobile, without tenderness. Supportive examinations were carried out in the form of transabdominal gynecological ultrasonography, alpha fetoprotein (AFP) and β hCG values. The ultrasound showed an anteflexed uterus measuring 7.9x2.73x3.77 cm, endometrial line (+) measuring 5.42 mm, showing a hypo hyperechoic picture with a size of 6.24x4.48x4.61 cm, the origin of the mass is difficult to assess, no ascites is found, the impression is in the form of intraabdominal mass (Figure 1). AFP value is 1.82 ng/ml. Furthermore, upon the finding of a growing mass, the patient was suspected of having a new intra-abdominal mass and planned a second look laparotomy.
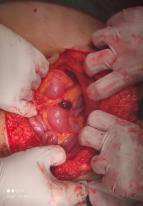
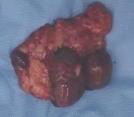
On exploration, we found the uterine and right ovary were within normal limit. It also was found 3 nodules on the peritoneum and omentum that conglomerated in the superior uterine, with the largest being 3x4 cm and the smallest being 2x2 cm (Figure 2), then adhesiolysis was performed and it was decided to take a mass and omentectomy for histopathological examination. Further exploration found nodules in the intestine measuring 2x2 cm, then a mass was taken for histopathological examination (Figure 3). Further exploration revealed no nodules in the liver. After the procedure, the patient underwent 2 days of treatment and went home after the second day of treatment. Histopathological results after surgery showed the results of glandular structures lined with cuboidal epithelial cells with cell nucleus morphology within normal limit. In the histopathological preparations, there were no signs leading to malignancy and no signs leading back to immature teratoma.
Discussion
Immature teratoma is a type of non-epithelial malignancy in the ovary. The degree of malignancy depends on the appearance of the neuroepithelium in the tumor. The incidence of this immature teratoma is about 10-20% among germinal malignancies of the ovary. Age up to 20 years is the largest population with this disease [4]. Immature teratomas occur generally in the first 2 decades of life and are mostly absent at menopause. This tumor was determined histologically based on the grade number and degree of immaturity of the cells [5]. The patient was first diagnosed with an immature teratoma at the age of 16 years with complaints of a palpable mass in his abdomen. Then the patient was decided to undergo a surgical procedure and the histopathological results of an immature teratoma were obtained. The growth of immature teratomas is quite fast with a median time between onset of symptoms and diagnosis of 5-12 weeks. The patients were present with complain of palpable pelvic mass, and abdominal pain. Experiences of other patients with abnormal vaginal bleeding were found 12% patients presented with abdominal pain, which may be associated with capsule rupture or twisting [6,7]. Immature teratomas consist of all germ cell networks, which is endoderm, mesoderm, and ectoderm and assessed histologically. The five-year survival rate of immature teratomas is around 90% in stage I and II but it descent to 82% for stage III and 72% for stage IV. High grade histology significantly get worsens the prognosis [8].
Incidence of immature teratoma on both ovaries at the same time is very rare. These tumors can have other implants in the surrounding tissue such as the peritoneum which is usually found intraoperatively. Prognosis is depending on the histological grade of the tumor and metastatic implants [9]. Peritoneal dissemination on immature teratoma mainly on the age of the children is a rare case and often provide a poor prognosis[10]. In the pediatric population with immature teratoma, elevated serum AFP levels were found in 50% of cases, but in adult there was found in one-third of cases. Levels of cancer antigen-125 (CA-125) were also found to have a less significant increase. Immature ovarian teratoma was not associated with elevated levels of human chorionic gonadotropin (hCG). Ultrasound may or may not describe the diagnosis of this disease, depending on the number of solid or immature tissue components [6].
In pre-menopausal patients in whom the tumor usually appears on one ovary, unilateral oophorectomy and limited grade surgery should be performed. Resection of other tumors that may cause morbidity and making delay for chemotherapy should not be performed. Resection of residual mass should be happened after completion of chemotherapy [4]. Patients with early-stage disease such as stage IA have a better prognosis, and adjuvant therapy after surgery is not required. In patients with high-grade, stage 1A immature teratoma, adjuvant chemotherapy is generally given, although this is sometimes questioned [4]. The commonly used combination chemotherapy regimen in the past was VAC (vincristin, actinomycin, and cyclophosphamide), however a study by the Gynecologic Oncology Group (GOG) reported that the relapse free rate in patients with incompletely resected disease was only 75%. Over past 20 years have incorporated cisplatin into the treatment of these disease, and mostly with VBP (vinblastine, bleomycin, and cisplatin) in the past and now with BEP (bleomycin, etoposide, and cisplatin) [4]. GOG has evaluated of three cycle BEP therapy in fully resected stage I, II, and III ovarian germ cell tumors patients. 91 of the 93 patients (97.8%) with non dysgerminomatous tumors were clinically disease free [4]. Administration of cisplatin can reduce the components of immature cells in teratoma derived from embryonic stem cells and pluripotent stem cells which are believed to cause apoptosis and also induce differentiation [2].
The second look laparotomy for ovarian germ cell tumor is not indicated for patients who have received adjuvant chemotherapy (eg, stages 1A, 2, and 3). However, this procedure is considered in suspected metastatic residual immature teratoma because it can lead to a rare complication such growing teratoma syndrome [4]. GTS was defined by Logothetis et al. in 1982 as a growth of benign tumor phenomenon, after removal of the primary malignant tumor during or after chemotherapy [11]. The incidence of GTS in testicular nonseminomatous germ cell is 1.9% to 7.6%, and occure in 12% of ovarian germ cell tumors [12,13]. GTS was determined based on the following criteria:
1. Continued growth of extra-gonadal tumor foci after diagnosis of immature teratoma or mixed germ cell tumor containing immature teratoma during or after chemotherapy with.
2. Serum markers (AFP and hCG) previously increased become normal and.
3. Histopathology shows mature teratoma in the resected tumors.6 Complete resection is important for the treatment of GTS. The presence of residual mass after surgery is a risk factor for recurrence GTS [14,15].
Patients who have undergone a laparotomy have a mass taken in the omentum and intestine. The histopathological results that we found did not find any malignancy in the nodule preparation. Preoperative findings with the presence of a mass after chemotherapy and a normal level of tumor marker AFP in the patient, two of the three GTS criteria proposed by Logotheis were met. The third condition that is met is the presence of a mature teratoma mass in a new mass histopathological preparation. In terms of histopathological results, this patient had histopathological results that did not show malignancy in the preparation. Treatment of GTS is multi-disciplinary approach, such as chemotherapy and surgery. GTS is diagnosed during or after the completion of chemotherapy. In case when GTS is recognized during chemotherapy, completion the early chemotherapy cycle is highly recommended. Discontinuation of chemotherapy is warranted only in the presence of vital symptoms caused by an increase in tumor size and in the presence of compression in surrounding organs (eg, intestine, lung, liver) with life- threatening organ failure. Only in this case, early resection of mass is acceptable. Improvements in oncological outcomes were achieved by following three steps:
1. Early recognition growing tumor size and elevated tumor markers during chemotherapy,
2. Completion of a number of early stages of the chemotherapy course under thorough monitoring of clinical size and appearance, and
3. Complete resection of growing mass [16,17].
According to Hamayun Imran et al. in his study stated that majority of GTS case have been reported in adults after adjuvant chemotherapy for all women except grade 1 tumor stage IA [18]. In a study conducted by Julie My Van Nguyen et al., stated that currently the incidence of GTS after adjuvant chemotherapy is becoming more common, as evidenced by the fact that there were 15 samples receiving adjuvant chemotherapy, 6 of whom had GTS so that further examination or required. The follow up was periodic patients with a history of immature teratoma through examination of tumor markers and imaging [19]. In a study conducted by Song Li et al., stated that GTS has a very good prognosis. Patients with GTS were found to be disease-free for 40.3 months (range 1-216 months, n=48) based on the median follow-up score. In addition, regular imaging and serum tumor markers follow-up are important [15]. GTS can develop in children, and the tumor size are same between adolescents and adults. Furthermore, GTS progression from primary germ cell tumors may be more rapid in children than adolescents and adults. Complete resection of all GTS tissue is recommended, although fertility sparing surgery should be considered whenever possible [20].
Conclusion
Immature teratoma can leave residual disease which will later become a rare complication, namely growing teratoma syndrome. Treatments of GTS are multi- disciplinary approach such as chemotherapy and surgery.




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.