Single Oral Dose Toxicity Test of Acorus Gramineus and Stachys Sieboldii Water Extracts and their Mixture in ICR Mice
Introduction
In recent times, with the increasing interest in health and well-being, public attention and demand for functional foods is growing. Herbal medicines and crude drugs are not only used as medicinal resources but also as major food resources, and the range and frequency of their use are gradually increasing [1,2]. This is resulting in a rise in the reckless use of functional foods and natural medicines made from various herbal and crude medicines, with a wide range of products and uses being available without appropriate regulations [3-5]. Due to the belief that natural material-based crude/herbal medicines would be safe because they have been used since a long time and for traditional oriental medicine and home remedies, scientific evidence on their toxicity and adverse effects has not been well established. Therefore, it is essential to lay the scientific foundation and verify the properties of these medicines [6,7]. In addition, in recent years, safety issues pertaining to the human bodies have been more important than ever before, and the value of functional materials with excellent efficacy cannot be well appreciated unless their safety has been confirmed [8,9]. Therefore, the safety of natural material-based crude drugs and herbal medicines should be consistently and systematically established. Accordingly, it is becoming critical to accurately evaluate the toxicity and adverse effects of active ingredients of extracted and purified natural materials using the latest standardized evaluation methods.
Stachys sieboldii Miq. is a herbaceous plant with tuberous stem belonging to the Stachy Linne genus in the Labiatae family [10]. The medicinal part of the root is a tuber-like part that appears like a bulb, which is typically 1–3 cm long and has a conch-like shape. It is described as a spiral shell-like silkworm in China and conch shell in Japan. Stachys sieboldii Miq. originated from China and came into cultivation in the 13th century. It is believed that it arrived in Korea through Japan and began to be cultivated [11,12]. Its root is used as an ingredient for general foods and health functional foods and its main constituents include chlorine; phenylethanoid derivatives such as martynoside and stachyose; and irioid derivatives such as meltoside, satchysoside A, harpagide, 8-acetylharpagide, starchyose, and acetoside, which have excellent antioxidative and anti-inflammatory properties [13]. In contrast, the pharmacological action and efficacy of Acorus gramineus Soland., a plant belonging to the Araceae family, have been reported in old books, such as Bonchogangmok and Donguibogam (Principles and Practice of Eastern Medicine), since ancient times. Acorus gramineus Soland. contains aromatic oils such as asaron, calameone, and eugenol, in addition to starch, acotin, tannin, vitamin C, and alkanoid; it has been known to be effective in improving memory [14], protecting brain cells [15], treating stroke [16], and improving blood lipid levels [17], among others.
According to a recent study, the combination of extracts of Gojiberry, Coix lacryma-jobi L., Alisma canaliculatum, and Astragalus propinquus has an impact on body weight, lipid metabolism, inflammation, and immune function [18], and it was reported that the combined administration of red ginseng and Gastrodia elata increased inhibitory effects on hyperlipidemia and vascular inflammatory diseases compared to their single administration [19]. As such, it has been confirmed that complex extracts increase or improve the effect of single extracts. Further, the use of various complex extracts has also been increasing. In particular, the physiological activities and potential uses of Stachys sieboldii Miq. and Acorus gramineus Soland. extracts have been investigated, but information on the safety and toxicity of their single and combined extracts is limited. In this study, to obtain data on the recently raised toxicity and safety issues caused by the abuse of herbal medicines and crude drugs, we performed a single-dose toxicity study on hot water extracts of Acorus gramineus Soland. (AGS) and Stachys sieboldii Miq. (SSM) and their combination, i.e., complex extract (MIX), using ICR mice to ensure their safety as functional natural materials.
Materials and Methods
Test Animals
SPF ICR mice at the age of 5 weeks obtained from OrientBio Inc. (Seongnam, Korea) were acclimated for 1 week at the animal breeding facility at Binary Inc. Among them, 6-week-old healthy male mice with 27.00 ± 0.96 g of body weight were selected and used in the study. The animals were maintained in a polycarbonate cage with ≤ 5 animals/cage, with the breeding environmental conditions of 23°C ± 3°C temperature, 30% ± 10% relative humidity, 12-hour light (08:00~20:00), and 150~300 lux illumination. The diet for experimental animals consisted of solid feed (OrientBio Inc.), and the water provided was prefiltered tap water. Food and water were provided ad libitum. This animal study was approved by the Institutional Animal Care and Use Committee (IACUC-2018-09) and performed following the approved procedures.
Preparation of Test Materials and Extracts
The dried Acorus gramineus Soland. and Stachys sieboldii Miq. were provided by Kwangdong Pharmaceuticals Inc. (Seoul, Korea). The dried AGS and SSM were extracted with hot water by 90 g each, filtered, and the solvent was removed using a rotary evaporator. After freeze-drying, 14.4% and 33.3% powders respectively were obtained based on dry weight. Individual AGS and SSM hot water extracts were prepared by suspending their powders in sterilized water. The combined/complex extract (MIX) was prepared by mixing them in a 1:1 ratio. Following this, single oral dose toxicity tests for each extract were performed.
Chromatographic Analysis
The two samples (AGS and SSM) was dissolved in 10 mg/mL 50% methanol; its phytochemical composition was analyzed using high-performance liquid chromatography (HPLC) with an Agilent 1260 series HPLC instrument (Agilent Technologies, San Jose, CA, USA) and an Agilent Extend-C18 column (250 × 4.6 mm). The column was operated in gradient mode with a mixture of 0.1% formic acid in water and acetonitrile as solvents (eluent B: 5–95% in 55 min), a flow rate of 1 mL/min, and an injection volume of 10 μL. The chromatograms were recorded at 254 nm and 320 nm, each peak was in the UV/visible spectrum (200–400 nm).
Dose Determination and Administration Method
Experimental group separation was performed on the last day of the acclimation period for all animals, and 60 selected animals were randomized into 8 animals per group to distribute for equal average body weight. As a pretest, two ICR mice were administered with 2,000 mg/10 ml/kg of each AGS, SSM, and MIX at a 2,000 mg/kg dose, which is the standard dose for nontoxic materials established by the US environmental protection agency (US EPA). No mortalities were observed. Hence, 2,000 mg/kg was set as the maximum dose, and a total of six groups, including 1,000 mg/ kg dose and control groups, were selected for the experiments. Since the expected intake route for clinical application of the test substances was oral, the oral administration method was used, and individual dose volumes were calculated based on body weight after fasting on the day of administration according to 10 ml/kg. All test animals were fasted for 12 hours before administration, and extracts were administered intragastrically using an oral gavage needle for oral administration. The control group was administered with the same amount of physiological saline as AGS, SSM, and MIX groups. Feed was restricted for 2 hours after administration, but drinking water was continuously supplied without restriction.
Clinical Signs and Body Weight Monitoring
Clinical signs were observed daily during the acclimation period of 7 days, every hour for 6 hours after administration on the day of AGS, SSM, and MIX administration, and at least once a day from days 1 to 14 after administration. Changes in general conditions, such as skin, hair, eyes, and mucous membranes, the onset of poisoning symptoms, mortality, and possible symptoms after administration were monitored. Also, body weight was measured just before administration and every other day from day 1 to 14 after administration.
Necropsy of Sacrificed Test Animals
The test animals were fasted for 12 hours the night before sacrifice and anesthetized using inhalational anesthesia. Blood collection was performed by laparotomy. The lesions of major internal organs that appear after blood collection and bleeding were visually observed, and histopathological examination was not performed because no gross abnormalities were observed during necropsy. Tissues including liver, heart, kidney, lung, spleen, testes, thymus, and brain were collected, washed ≥3 times with physiological saline, drained, and weighed. For bilateral organs, weights of both sides were measured.
Hematological Analysis
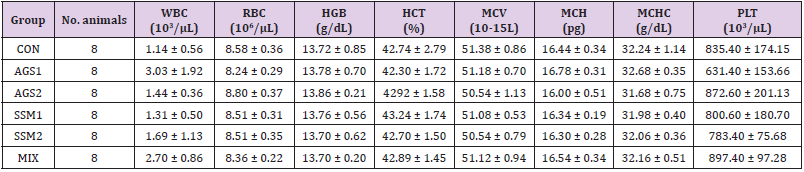
Hematological analysis included complete blood count using hematology analyzer (Coulter counter, Coulter Co., Miami, FL., USA), white blood cell count (WBC), red blood cell count (RBC), hemoglobin levels (HGB), mean corpuscular volume (MCV), mean corpuscular hemoglobin levels (MCH), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), etc.
Blood Chemistry Analysis
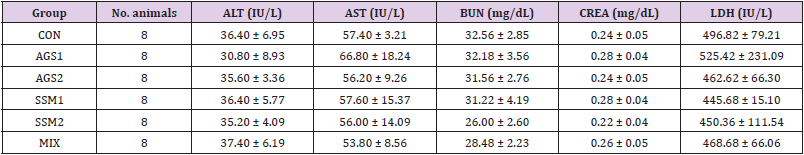
For blood chemistry analysis, the collected blood was allowed to coagulate for least 30 minutes and then centrifuged at 3,000 rpm for 10 minutes to separate the serum, followed by measurement of aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN), creatinine (CREA), and lactate dehydrogenase (LDH) using an automated blood chemistry analyzer (Prestige 24i, Tokyo Boeki Medical System Ltd., Tokyo, Japan).
Statistical Analysis
All results were represented in mean ± standard deviation, calculated using SPSS ver. 22.0 (SPSS Inc., Chicago, IL, USA). To verify the statistical significance for each analysis item of each experimental group, analysis of variance was performed. The Student’s t-test and Duncan’s multiple range test were used to verify the significance of p < 0.05.
Results
Chemical Characterization of AGS and SSM
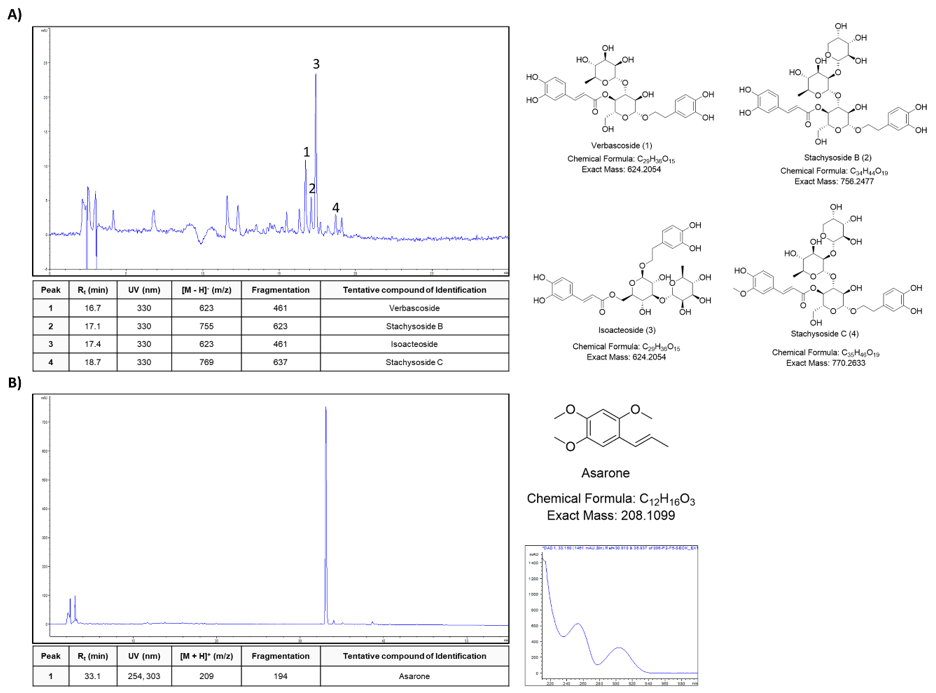
We used HPLC and LC-MS/MS with ESI to characterize the AGS and SSM extracts. Each major peaks were identified in the HPLC profile of the AGS and SSM extracts. The identification of the chemical compounds was also carried out by comparing the molecular ion peaks along with the MS fragmentation pattern with those of the literature [20]. As shown in (Figure 1A), AGS extract Peaks 1, 2, 3, and 4 were tentatively identified as Verbascoside, Stachysoside B, Isoacteoside, and Stachysoside C, respectively. In addition, SSM extract major peak was tentatively identified as Asarone (Figure 1B).
Figure 1: Fingerprint analysis of AGS and SSM extracts. HPLC and LC-MS/MS analysis of the major compounds from
(A) AGS and
(B) SSM extracts, respectively.
Mortality Rate and LC50 Value
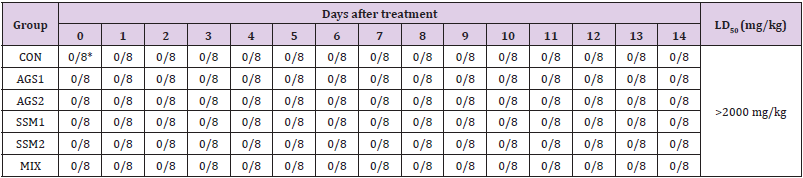
The results of toxicity signs and mortalities caused by SSM, AGS, and MIX in ICR mice are presented in (Table 1). From the result of the 14-day observation period of the treatment group receiving a single oral administration of 1,000 and 2,000 mg/kg doses of AGS, SSM, and MIX and the control group receiving a single oral administration of sterile physiological saline, no mortalities were noted in all groups, including the highest dose group. Therefore, the minimum lethal dose of AGS, SSM, and MIX exceeds 2,000 mg/kg in ICR mice. In addition, lethal concentration 50 (LC50) of AGS, SSM, and MIX is estimated to be over 2,000 mg/kg.
Table 1: Mortality of ICR mice orally administered with AGS, SSM, and MIX.
Note: CON; Control group, AGS1; AGS 2,000 mg/kg (day) medication group, AGS2; AGS 1,000 mg/kg (day) medication group, SSM1; SSM 2,000 mg/kg (day) medication group, SSM2; SSM 1,000 mg/kg medication group, MIX; MIX 2,000 mg/kg (day) medication group. *Values are expressed as Number of dead animals/ Number of animals examined.
Drinking, Feed Intake and Clinical Signs
After comparing the changes in drinking yield and feed intake by single oral administration of AGS, SSM, and MIX in the treatment groups with the control group receiving a single oral administration of sterile physiological saline, administration of the test substances did not result in any significant differences in the changes in drinking yield and feed intake (data not shown). Further, no abnormal findings in clinical symptoms related to single oral administration of sterile physiological saline, AGS, SSM, and MIX were observed, including hair loss, activity decline, gait disorder, behavior disorder, squat, diarrhea, swelling, dyspnea, grooming, jumping, tearing, lethargy, polyuria, vomiting, nasal discharge, numbness, suppleness, etc. (data not shown).
Changes in Bodyweight
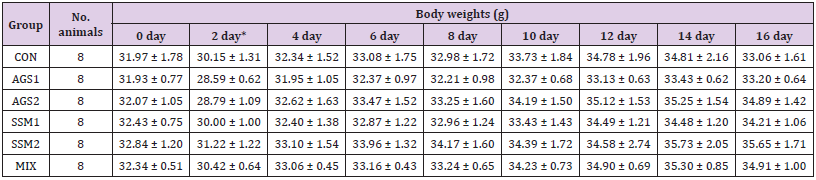
The results of changes in bodyweight of the treatment and control groups are presented in (Table 2). After oral administration, normal weight gain over time was observed in the AGS, SSM, and MIX administration groups and the control group compared to the weight before administration. No significant weight change was noted after administration, compared to that before administration, in the treatment (AGS, SSM, and MIX administration) and control groups, indicating no toxicity.
Table 2: Body weights changes of ICR mice orally administered with AGS, SSM, and MIXb.
Note: CON; Control group, AGS1; AGS 2,000 mg/kg (day) medication group, AGS2; AGS 1,000 mg/kg (day) medication group, SSM1; SSM 2,000 mg/kg (day) medication group, SSM2; SSM 1,000 mg/kg medication group, MIX; MIX 2,000 mg/kg (day) medication group. The data are presented as mean ± standard deviation. *Day after AGS, SSM, and MIX administration.
Necropsy Results and Change in Organ Weight
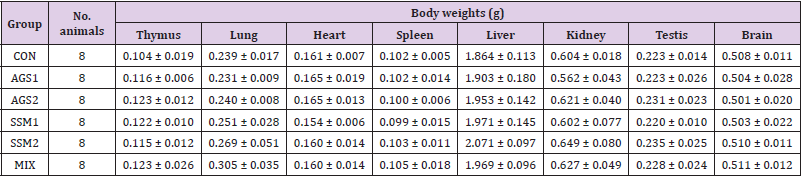
The results of gross findings on major organs by necropsy of all ICR mice after the 14-day observation period are presented in (Table 3). There were no gross abnormalities or abnormal lesions on major internal organs suspected of causing abnormalities by the administration of test substances in all animals in the control and treatment groups. In addition, no significant changes in the weights of the thymus, lungs, heart, spleen, liver, kidney, testes, and brain were observed in the treatment groups compared with that in the control group.
Table 3: Organ weights of ICR mice orally administered with AGS, SSM, and MIX.
Note: CON; Control group, AGS1; AGS 2,000 mg/kg (day) medication group, AGS2; AGS 1,000 mg/kg (day) medication group, SSM1; SSM 2,000 mg/kg (day) medication group, SSM2; SSM 1,000 mg/kg medication group, MIX; MIX 2,000 mg/kg (day) medication group. The data are presented as mean ± standard deviation.
Hematological Analysis
The evaluation of WBC, RBC, HGB, HCT, MCV, MCH, MCHC, and PLT using a hematological analyzer was used to investigate hematological changes 14 days after oral administration of either sterile physiological saline, AGS, SSM, or MIX. The results of this evaluation are shown in (Table 4). From the results of hematological analysis on collected whole blood from the treatment and control groups, PLT in the groups treated with AGS, SSM, and MIX showed slight reduction compared to the control group, albeit not significantly. The other categories showed no significant changes between the control group and the treatment groups.
Table 4: Hematological analysis of ICR mice orally administered with AGS, SSM, and MIX.
Note: CON; Control group, AGS1; AGS 2,000 mg/kg (day) medication group, AGS2; AGS 1,000 mg/kg (day) medication group, SSM1; SSM 2,000 mg/kg (day) medication group, SSM2; SSM 1,000 mg/kg medication group, MIX; MIX 2,000 mg/kg (day) medication group. The data are presented as mean ± standard deviation.
Blood Chemistry Analysis
The results of serum ALT, AST, BUN, CREA, and LDH values measured using an automated blood chemistry analyzer for investigating blood biochemical changes after 14 days in the treatment and control groups are shown in (Table 5). It was found that a single oral administration of AGS, SSM, and MIX induced a slight change in the test parameters, but in general, no significant changes were observed in all indicators between the control group and the treatment groups.
Table 5: Blood chemistry analysis of ICR mice orally administered with AGS, SSM, and MIX.
Note: CON; Control group, AGS1; AGS 2,000 mg/kg (day) medication group, AGS2; AGS 1,000 mg/kg (day) medication group, SSM1; SSM 2,000 mg/kg (day) medication group, SSM2; SSM 1,000 mg/kg medication group, MIX; MIX 2,000 mg/kg (day) medication group. The data are presented as mean ± standard deviation.
Discussion
Recently, various types of medicines are being used, but problems such as adverse effects due to toxicity also appear. Not only is the interest in functional foods and natural medicines using herbal medicines and crude drugs is increasing worldwide but also their effect and efficacy are being verified, owing to an increasing demand for various forms of natural-product derived pharmaceuticals [21,22]. However, in the general practice of natural medicine, which prescribes a combination of various crude drugs, exact ingredients and specifications are not well established and data on their safety and toxicity are often insufficient, thereby necessitating specific and accurate information on them [6,7,23]. Therefore, in this study, to obtain an objective basis for the safety of AGS, SSM, and MIX and experimentally evaluate their acute toxicity, the observation of clinical symptoms, necropsy findings, mortality, and weight change, and hematological analysis were conducted after administering the test substance to ICR mice. First, an acute toxicity test was performed to confirm the safety of AGS, SSM, and MIX, following which all subjects in the treatment groups treated with AGS, SSM, and MIX as well the control group treated with sterile physiological saline showed no mortality and no significant change in body weight.
Therefore, based on the US EPA standards that classify a substance safe if its LD50 value by oral administration is >2,000 mg/ kg, AGS, SSM, and MIX are considered to be very safe in terms of acute toxicity. Next, from the result of gross examination and organ weight measurement by necropsy to confirm the effects of AGS, SSM, and MIX on the major internal organs, no gross abnormalities or abnormal lesions were observed and no significant changes in the weights of major organs, such as the thymus, lungs, heart, spleen, liver, kidney, testes, and brain, were observed. In general, in a single-dose toxicity study, if gross abnormalities in organs or tissues are observed, histopathological examination should be performed. However, in this study, histopathological examination was not performed as no gross abnormalities were observed in all experimental animals. Also, hematological and blood chemistry analyses using whole blood and serum, respectively, collected at the end of the observation period revealed slight changes in some test parameters, but in general, no significant changes were noted in terms of AGS, SSM, and MIX treatment in all test parameters. In summary, as AGS, SSM, and MIX did not show any acute toxicity on the test animals, they could be considered relatively safe for oral administration. In addition, they can be expected to be used as natural materials without acute toxicity through further investigation of their physiological effects.
However, there are some limitations of determining the toxicity of natural herbal medicines through only a single oral administration acute toxicity study. Hence, it is necessary to conduct repeated oral administration toxicity studies for additional 2 or 4 weeks and 13 weeks (long term) and genotoxicity studies subsequently. In addition, further research on human safety evaluation is essential, based on which more precise and scientifically accurate safety data can be obtained by establishing systematic toxicity information on AGS, SSM, and MIX.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.