Successful Desensitization to Dacarbazine Using Pretreatment with Omalizumab
Introduction
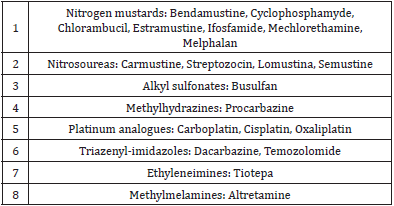
Dacarbazine (DTIC) is an antineoplastic drug that belongs to the group of the alkylating agents (they destroy cells by attaching an alkyl group (CnH2n+1) to their DNA) (Table 1). It is used to treat several cancer types (i.e., melanoma, Hodgkin lymphoma, pancreatic islets carcinoma and some types of sarcoma). It is often administered in combination chemotherapy, for example in the ABVD regimen used in the first-line treatment of Hodgkin lymphoma, in which A stands for Adriamicine, B for Bleomicine, V for Vinblastine and D for Dacarbazine. Dacarbazine-induced adverse drug reactions (ADR) can be caused by several mechanisms, i.e., drug interactions when it is given simultaneously to other drugs that are metabolized through the cytochrome P450 -this liver enzyme is needed for the activation of dacarbazine by demethylation. Hypersensitivity reactions seem to be infrequent when we look at the scarce number of published papers regarding this subject. Different types of reactions have been described, both immediate (anaphylactic shock, urticaria) and delayed (hypersensitivity syndrome, allergic hepatotoxicity, photosensitivity -phototoxic dermatitis-, fixed drug eruption, eosinophilia) [1-5].
Even if an IgE-mediated mechanism has not been established, the clinical picture of some of the immediate reactions in which dacarbazine is involved suggests that they could be caused by this immunological mechanism. We must take into account that the dacarbazine formulation available in Spain contains mannitol as an excipient, which could have a role in causing some of the dacarbazine-induced ADR, acting as an allergen itself or by means of a non-immunological mechanism (for example, inducing histamine release when it is given intravenously as hyperosmolar solutions) [6-8].
Case Report
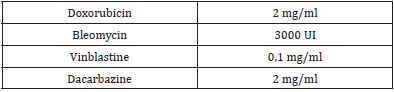
We report the case of a 19-year-old man diagnosed with Hodgkin’s disease who presented an immediate reaction after receiving treatment with doxorubicin (adriamicine), bleomycin, vinblastine and dacarbazine. He had an atopic background: he had previous diagnosis of chlorhexidine allergy (generalized urticaria after its topical use), atopic dermatitis in childhood, kiwi allergy (oral allergy syndrome) and allergic rhinitis with grass pollen and cat sensitivity. The reaction during the administration of chemotherapy started with an itchy feeling of his throat and the base of the tongue that progressed to respiratory distress, generalized urticaria and facial angioedema. The symptoms resolved within an hour after he was given 100 mg of hydrocortisone. It was the first time that he received this treatment. Skin prick tests with doxorubicin, bleomycin, vinblastine and dacarbazine were empirically performed (Table 2); we obtained a doubtful positive result with dacarbazine. We did not perform intradermal tests because of the vesicant effect that has been described with some of these drugs (doxorubicin, vinblastine) and also because we didn´t find published concentrations for such tests with these drugs.
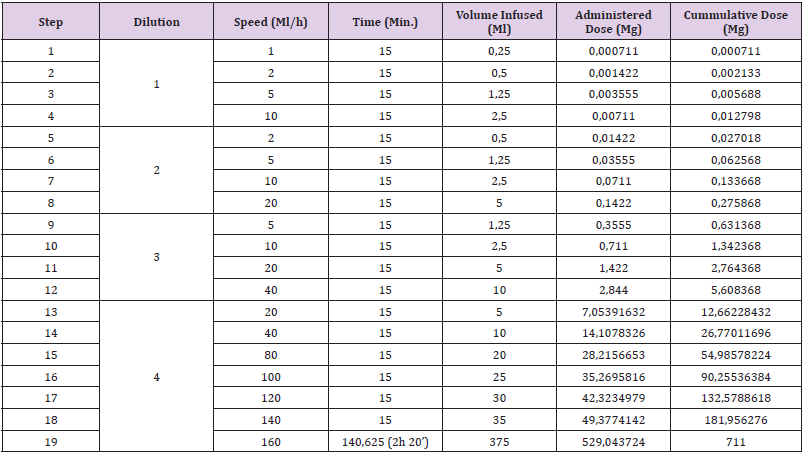
Given the results of the tests and the signs and symptoms presented by the patient, a 19 step, 4 concentrations (Table 3), desensitization protocol was performed in the Intensive Care Unit (ICU) of our hospital. The patient was given premedication with 8 mg of dexamethasone, dexchlorpheniramine and ondansetron. During the infusion of the last bag, he complained of pruritus in the base of his tongue and dyspnea. He was treated with dexchlorpheniramine and 100 mg of hydrocortisone, and the infusion was continued at a 30 ml/h speed. At that moment, he developed a systemic urticaria. It resolved with a new dose of dexchlorpheniramine and 100 mg of hydrocortisone. We stopped the procedure; so we couldn’t achieve the desired the full dose. The next desensitization procedure was also performed at the ICU; this time the patient was premedicated with a single dose of 300 mg of Omalizumab, given three weeks before the desensitization. The patient took at home acetylsalicylic acid 300 mg, montelukast 10 mg, prednisone 30 mg and cetirizine 10 mg the previous two days to the desensitization day, and acetylsalicylic acid 300 mg and montelukast 10 mg on the third day.
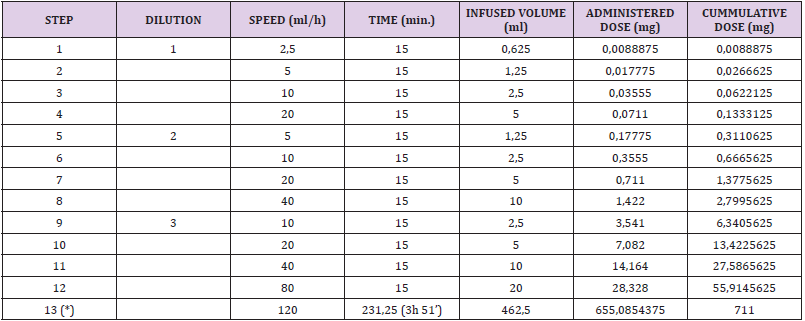
Table 3: first desensitization with dacarbazine protocol.
Note: Dilution 1: 0,0028 mg/ml (0,14 mg/50 ml)
Dilution 2: 0,0284 mg/ml (1,42 mg/50 ml)
Dilution 3: 0,2844 mg/ml (28,44 mg/100 ml)
Dilution 4: 1,4108 mg/ml (705,39 mg/500 ml)
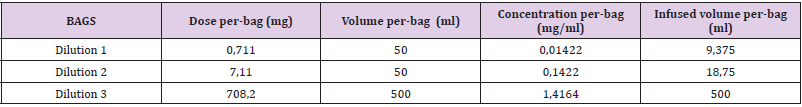
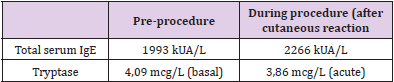
Blood samples were obtained before the procedure in order to get basal values of tryptase and total IgE and the hospital premedication was modified; he was given intravenous dexchlorpheniramine, 50 mg of ranitidine, 8 mg of ondansetron and 16 mg of dexamethasone. A 3 bag, 13 step protocol was followed (Tables 4-6). About 90 minutes after the last infusion speed (120 ml/h) was started, he presented a few isolated, non-pruritic hives that were treated with intramuscular injection of 40 mg of methylprednisolone, without stopping the desensitization. New blood samples were drawn and dexchlorpheniramine was added; the procedure could be successfully finished. One hour after the desensitization had finished the skin lesions disappeared. Subsequent desensitizations were performed with the administration of omalizumab one week before each new treatment cycle (it was given every two weeks). The previously described 13 step regime was followed, although a new dose of dexchlorpheniramine was added before the change to the 120 ml/h infusion speed. Hitherto, he has tolerated all the desensitizations.
Table 5:
Note: (*) Before step 13 dexchlorpheniramine is given
Total duration of the procedure: 6h 51’
Discussion
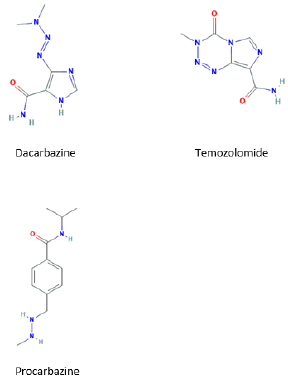
Previous desensitizations to dacarbazine have been described, but they represent a tiny part of the procedures that have been performed to other antineoplastic drugs; we haven’t found descriptions of skin testing with this drug [9,10]. As we don’t know which concentration of dacarbazine causes irritation during skin testing, we think that the drug concentration we used for the skin prick test (2 mg/ml) requires further validation, testing it in, ideally, at least 10 controls (which we didn´t perform). Patients who develop an allergy to dacarbazine could react to the administration of temozolomide, because of the similar molecular structure of these molecules (Figure 1). This could be troublesome for melanoma patients, because both drugs are therapeutic options in these patients. Anyway, it’s an unlikely event, because temozolomide is mainly used in cerebral tumors. Besides, even if cross reactivity between these two drugs was possible, their different route of administration (dacarbazine is given intravenously and temozolomide per os) may cause different clinical reactivity [5].
Both drugs, and also procarbazine, produce as intermediate agents alkyl diazonium derivatives, which are responsible of the alkylation of biologic molecules. It has yet to be stablished if these products can have a pathogenic role in the adverse reactions to these drugs. Our patient had a serious reaction during his first exposure to dacarbazine. The mechanism involved in the reaction could be immunological or non-immunological. If we consider a possible immunological mechanism, the previous sensitization could have happened by contact with molecules structurally similar to dacarbazine or by developing a sensitization to mannitol, the excipient used in the dacarbazine commercial formulation. Mannitol is a ubiquitous agent, often used in food and pharmaceutical industry. One of the main limitations of our study is that we didn’t perform skin tests with mannitol; the other, the fact that we didn´t test the concentrations we used for prick test in controls.
Considering the likelihood of an IgE-mediated mechanism in the dacarbazine-induced reaction, it would be necessary to have validated and reliable skin tests to obtain a diagnosis, especially in patients like ours who have their reaction in the context of the simultaneous administration of multiple antineoplastic drugs, thus making very challenging the identification of the culprit drug which will probably have to be readministered using a desensitization protocol. Also, knowing whether there is or there isn’t an immunological sensitization can help us stratify better the risk in order to choose the proper protocol in the desensitization [10,11]. As it has been described elsewhere, omalizumab is useful as an adjuvant therapy in the desensitizations that are unsuccessful with the standard protocols [12,13].
For more Articles on: https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.