Study on the Secondary Metabolites from a Plant- Derived Streptomyces SP. D-154
Introduction
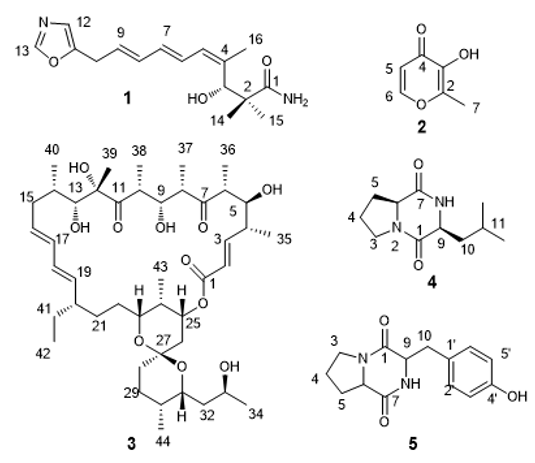
Agricultural antibiotics is considered to be one of the important biopesticide to protected agricultural plants from insect pests. Microorganism have produced many famous agricultural antibiotics like kasugamycin, Jinggangmycin, agricultural antibiotic 120 and abamectin [1]. Nowadays, more and more agricultural antibiotics lose their potency over time due to the development of resistance. So we need exploited more antibacterial or insectresistant candidate compounds have novel structures and lower toxicities[2]. It’s become harder and harder getting new bioactive substances from soil microbial with the broad research nearly half a century. Another less studied microbial taxa endophytic bacteria isolated novel and activity substances is increasingly attracted people’s attention [3]. The object of this study was Streptomyces sp. d-154, a strain of naoyanghua collected from Kunming Botanical Garden, Yunnan Province. In the previous activity screening experiment of a small amount of fermentation broth extract, we found that the extract had obvious antibacterial activity. Therefore, five compounds were obtained by mass fermentation of the strain and extraction and separation of secondary metabolites in the extract of fermentation broth. Through the analysis of physical and chemical properties and spectral number, they were identified as inhomycin B (a), maltol (b), rutamycin (c), cyclo (l-pro-l-leu) (d) and cyclo (l-pro-l-tyr) (e) (Figure 1). Compound 3 showed certain antifungal activity.
Biological Material
Strain Streptomyces sp. KIB-D154 was isolated from a Rhododendron molle sample obtained from Kunming Botany Garden, Kunming, China. The organism was isolated using the standard dilution plate method and grown on Gause′s agar medium at 28 oC for 8 days.
Fermentation and Isolation
The seed solution were carried out in 250 mL baffle Erlenmeyer flasks. Each flask was filled with 50 mL of Tryptone Soy Broth (30g/L) and cultivated for 2 days at 28 oC on a rotary shaker (250 rpm). The flasks were inoculated with 10 ml seed solution and cultivated for 5 days at 28 oC on a rotary shaker (250 rpm). Fermentations were carried out in 1000 mL baffle Erlenmeyer flasks. Each flask was filled with 250 mL of medium consisting of Trypton 0.02%, yeast extract 0.02%, soluble starch 0.12%, D-glucose 0.05%, corn steep liquor 0.07%, soybean powder 0.075%, K2HPO4 0.05%, MgSO4﹒7H2O 0.05%, CaCO3 0.02% and NaCl 0.04% in deionized H2O (pH 7.2-7.5). A 6 L fermentation broth was centrifuged (6000 rpm, 20 min) and the liquid supernatant was extracted with ethyl acetate (3 × 4 L), and the mycelium was extracted with acetone (3 × 0.5 L). Both parts were combined. The solvent was removed by evaporation, and the residue was subjected to RP-18 chromatography.
Elution with H2O/MeOH (gradient from 30% to 100% MeOH, column 50 × 3 cm) yielded six fractions A to F. Fraction E was applied to semipreparative HPLC (HITACHI HPLC system; YMCTriart C18 column, 250 × 10 mm; DAD detector) with a flow rate of 3 mL/min and 80% CH3OH in H2O led to 3 (14.3 mg). Fraction A separated by Sephadex LH-20 chromatography (MeOH, column 80 × 2 cm) led to 2 (1.3 mg), 4 (19.2mg) and 5 (9.1 mg). Fraction B was subjected to preparative HPLC using a Kromasil C18 column (10 μm, 100 Å) with a flow rate of 12 mL/min and UV detection at 430 nm. Isocratic elution with 30% MeCN in H2O afforded 1 (4.5 mg).
Antibacterial Assay
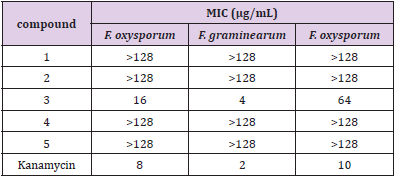
The minimal inhibitory concentration (MIC) of compounds 1-5 against Fusarium oxysporum f. sp. vasinfectum, Fusarium graminearum. Schw and Fusarium oxysporum was determined by microdilution method [4]. In the test, each compound was prepared into 128, 64, 32, 16, 8, 4, 2, 1, 05, 0.25 and 0.125 respectively(μg/ mL). Take the concentration of 11 suspension g / ml as the control, and take the positive suspension as the control [4].
Compound 1, White Amorphous Powder
C16H22N2O3, ESI-MS m/z: 291 [M+H]+, 1H NMR (CD3OD, 600 MHz) δ: 8.23 (1H, s, H-13), 7.09 (1H, s, H-12), 6.89 (1H, d, J = 9.5 Hz, H-6), 6.56 (1H, dd, J = 11.5, 14.0 Hz, H-8), 6.22 (1H, m, H-7), 6.08 (1H, d, J = 11.5 Hz, H-5), 5.92 (1H, d, J=17.1 Hz, H-9), 5.74 (1H, m, H-10),5.43(1H, s, 3-OH), 4.56 (1H, d, J =4.4 Hz, H-3), 1.70 (3H, s, H-16), 1.06 (3H, s, H-14), 0.92 (3H, s, H-15);13C NMR (CD3OD, 600 MHz) δ: 179.1 (C-1), 151.4 (C-11), 150.5 (C-13), 139.3 (C-4), 133.1 (C-9), 130.9 (C-8), 128.5 (C-7), 128.4 (C-6), 127.5 (C-5), 122.2 (C-12), 73.5 (C-3), 45.6 (C-2), 28.1 (C-10), 24.5 (C-14), 21.5 (C- 15), 19.8 (C-16).It’s spectral data and physicochemical data were identical to those recorded for inthomycin B [5].
Compound 2, Brown Oil
C6H8O3, ESI-MS m/z: 129 [M+H]+;1H NMR (CDCl3, 600 MHz) δ: 7.70 (1H, d, J = 5.0 Hz, H-5), 6.41 (1H, d, J = 4.3 Hz, H-5), 2.36 (3H, s, H-7);13C NMR (CDCl3, 600 MHz) δ: 172.9 (C-4), 154.2 (C-6), 148.7 (C-2), 143.1 (C-3), 112.8 (C-5), 14.2 (C-7). It’s spectral data and physicochemical data were identical to those recorded for maltol [6].
Compound 3, White Powder
C44H72O11;ESI-MS m/z: 777 [M+H]+;1H NMR (CDCl3, 400 MHz) δ: 6.62 (1H, dd, J = 15.6, 10.0 Hz, H-3), 6.03 (1H, m, H-17), 5.93 (1H, m, H-18), 5.81 (1H, d, J = 15.6 Hz, H-2), 5.46 (1H, t, J = 10.8 Hz, H-16), 5.25 (1H, dd, J = 14.3, 9.1 Hz ,H-19), 4.03 (1H, d, J = 14.0 Hz , H-25), 4.02 (1H,m, H-31), 3.97 (1H, m, H-9), 3.96 (1H, m, H-13), 3.82 (1H, d, J = 8.7 Hz, H-23), 3.77 (1H, d, J = 10.1 Hz, H-5), 3.62 (1H, m, H-8), 3.35 (1H, m, H-33), 2.76 (1H, m, H-10), 2.72 (1H, d, J = 7.4 Hz ,H-6), 2.38 (1H, d, J = 6.4 Hz , H-4), 2.20 (1H, d, J = 14.2 Hz ,H-15), 2.15 (1H, m , H-24), 2.12 (1H, m, H-29), 1.98 (1H, m, H-15), 1.93 (1H, m, H-14), 1.86 (1H, m, H-20), 1.78 (1H, m, H-26), 1.75 (1H, m, H-28), 1.66 (1H, m, H-32), 1.60 (1H, m, H-30), 1.59 (1H, m, H-28), 1.53(1H, m, H-22), 1.47(1H, m, H-22), 1.41 (1H, m, H-29), 1.40 (1H, m, H-21), 1.38 (1H, m, H-41), 1.32 (1H, m, H-41), 1.31(1H, m, H-32), 1.28(1H, m, H-21), 1.24 (3H, d, J = 6.0 Hz, H-35), 1.14 (3H, s, H-39), 1.11 (3H, d, J = 6.8 Hz, H-37), 1.07 (3H, d, J = 7.2 Hz ,H-36), 1.04 (3H, d, J = 6.9 Hz , H-40), 1.00 (3H, d, J = 6.4 Hz, H-38), 0.98 (3H, d, J = 6.3 Hz ,H-44), 0.97 (3H, d, J = 7.0 Hz , H-34), 0.83 (3H, d, J = 6.9 Hz ,H-43), 0.80 (3H, d, J = 7.5 Hz , H-42),;13C NMR (CDCl3, 400 MHz) δ: 220.5 (C-7), 220.1(C-11), 164.9 (C-1), 148.7(C-3), 137.9 (C-19), 132.6 (C-17), 130.5 (C-18), 129.6 (C-16), 122.8 (C-2), 97.5(C-27), 83.1 (C-12), 73.1 (C-5), 72.7 (C-9), 72.3(C-13), 70.9 (C-25), 69.9(C- 23), 67.5 (C-31), 64.7 (C-33), 46.7 (C-6), 46.0 (C-20), 45.8 (C-10), 42.7 (C-32), 42.0 (C-8), 40.2 (C-4), 38.6 (C-15), 35.7 (C-24), 35.5 (C-26), 33.6 (C-14), 31.4 (C-21), 31.0 (C-22), 30.7 (C-30), 28.7 (C- 41), 26.6 (C-29), 24.9 (C-28), 20.9 (C-39), 17.8 (C-35), 14.5 (C-40), 14.0 (C-37), 12.1 (C-42), 11.3 (C-44), 9.9 (C-34), 9.3 (C-38), 8.3 (C- 36), 5.3 (C-43). It’s spectral data and physicochemical data were identical to those recorded for rutamycin [7].
Compound 4, Colorless Crystal
C11H18N2O2;ESI-MS m/z: 211 [M+H]+;1H NMR (CDCl3, 400 MHz) δ: 6.53 (1H, s, 8-NH), 4.09 (1H, t, J = 8.0 Hz, H-6), 3.99 (1H, d, J = 7.6 Hz, H-9), 3.62 - 3.45 (2H, m, H-3), 2.31 (1H, m, H-5), 2.15 - 2.03 (2H, m, H-10), 1.93 (2H, m, H-4), 1.90 (1H, m, H-5), 1.51 (1H, m, H-11), 0.97 (3H, d, J = 6.4 Hz, H-13), 0.92 (3H, d, J = 6.4 Hz, H-12) ;13C NMR (CDCl3, 400 MHz) δ: 170.4 (C-1), 166.2 (C-7), 58.9 (C-6), 53.3 (C-9), 45.4 (C-3), 38.4 (C-10), 28.0 (C-5), 24.5 (C-11), 23.2 (C- 13), 22.7 (C-4), 21.2 (C-12). It’s spectral data and physicochemical data were identical to those recorded for cyclo(L-Pro-L-Leu ) [8].
Compound 5, Yellow Solid
C14H16N2O3;ESI-MS m/z: 261[M+H]+;1H NMR (CDCl3, 400 MHz) δ: 7.04 (2H, s, H-2′, 6′), 6.78 (2H, s, H-3′, 5′), 5.96 (1H, s, 8-NH), 4.22 (1H, d, J=8.0 Hz,H-9), 4.07 (1H, t, J=15.7 Hz, H-6), 3.69 - 3.59 (1H, m, H-3), 3.55 (1H, t, J = 9.2 Hz, H-3), 3.45 (1H, m, H-10), 2.83 - 2.73 (1H, m, H-10), 2.39 - 2.29 (1H, m, H-5), 2.05 - 1.97 (1H, m, H-5), 1.91 (2H, dd, J = 19.1, 13.9 Hz, H-4);13C NMR (CDCl3, 400 MHz) δ: 169.7 (C-1), 165.2 (C-7), 155.7 (C-4′), 130.3 (C-2′,6′), 126.8 (C-1′), 116.1 (C-3′, 5′), 59.1 (C-6), 56.2 (C-9), 45.4 (C-3), 35.9 (C-10), 28.2 (C-5), 22.4 (C-4). It’s spectral data and physicochemical data were identical to those recorded for cyclo(L-Pro-L-Tyr) [9].
Antibacterial Activity of Yellow Solid Compounds
In the antibacterial sensitivity test, only compound 3 showed certain antibacterial effect on three plant pathogenic fungi: F. oxysporum f. sp. vasinfectum, F. graminearum Schw and F. oxysporum, and the MIC values were 16, 4 and 64μg/mL, respectively. Other compounds did not show obvious inhibitory effect (Table 1).



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.