Analytical Quality by Design: A Review for Chromatography
Introduction to Quality by Design (QBD)
The pharmaceutical industry develops a high-quality product that is both safe and efficient. Not only does QbD improve the quality of the final product, but it also improves the quality of the entire production process. Finally, the product must satisfy patient’s demands as well as experimental results [1]. Various regulatory bodies are concerned with product safety. Manufacturing costs can be reduced by adopting PAT, CQA, and ATP. The strategies for developing goods vary from industry to industry. The substance is of high quality since it is contamination-free, resulting in a proper therapeutic effect [2]. For the development of a product, either an empirical or a systematic method, or both, can be employed; however, when implementing QbD, the systematic approach is used more [3]. A systematic approach facilitates designing the production process and accelerates formulation development, which results in a product worthy of being evaluated in a clinical trial. For goods with minimal risk of chemical, manufacturing, and control (CMC) modifications in post-approval manufacture, the FDA wants to reduce the regulatory filing requirement [4].
Design
i. Product should fulfil a patient’s demands.
ii. Product should be of good quality.
iii. The starting unprocessed materials will affect the product.
Definition of Quality by Design (QBD)
A systemic approach to development begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management.
Benefits of QBD
i. It improves the Quality of Product.
ii. It provides a better understanding of manufacturing.
iii. It increases production.
iv. Chances of batch failure are less.
v. The investigations are not costly [5-7].
vi. It reduces the time for manufacturing.
vii. The deviation is minimized.
viii. Less time is required.
ix. It gives a better understanding of manufacturing processes.
x. It reduces the time for testing.
xi. It builds patient’s confidence [8,9].
xii. It provides a positive environment.
xiii. The economic rate is high.
It is for better development.
Opportunities
i. It is efficient and flexible.
ii. It validates the process.
iii. It increases efficiency and potency.
iv. It reduces costs [10].
v. The speed for production is more.
vi. It reduces batch rejections.
vii. Scientific knowledge is more for all products.
viii. For various issues, better interact with industry,
ix. Chance of risk management is less [11].
Steps involved in QbD
The Target Product Quality Profile (TPQP): TPQP has been intended for quality of a drug product that will reach to secure the good product TPQP is defined as ,“prospective and dynamic summary of the quality characteristics of a drug product that ideally will be achieved to ensure that the desired quality, and thus the safety and efficacy, of a drug product is realized”. Thus safety, quality and efficacy of a drug is perceived. It cover strength of dosage form (s), route of administration of drug, therapeutic index , and pharmacokinetic parameters such as aerodynamic performance , Drug dissolution , relevant quality product its dosage form has been developed and product quality criteria such as purity and sterility suitable for the design of sells product [12].
Critical Quality Attribute: After the TPQP the next step involved is Critical quality attributes. CQA has been defined as “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distributed to ensure the desired product quality”. As per the ICH Q9 [12]. Guidelines identification of CQAs is done by risk assessment. For making risk assessments the knowledge of product required such as accumulated laboratory, clinical and non-clinical experience with certain product-quality attribute.
Critical Process Parameter: “Parameters whose variability have an impact on a CQA and therefore should be monitored or controlled to ensure the process produces the desired quality” are defined as Critical process parameters (CPPs). The ability of a process to demonstrate acceptable quality and performance and tolerate variability in inputs at the same time is defined as Process robustness. Process capability Should be considered. To indicate the uniformity and reproducibility of a process. The Six Sigma is popularly accepted for process capability. Process capability index is the statistical a process ability to build output within specific range [12]. Process capability index (CpK) = Upper limit of specification - Lower limit of specification / 6 standard deviation.
Risk Assessment: Quality risk management is a systemic approach for a control, communication and risk of a drug product quality of a lifecycle. The parameter that can affect CQAs can be reduced by QRA (Quality risk assessment. QRA is a process that can recognizes CPPs and lead to remove risk. Thus, Quality product achieved. By using QRA many parameters can remove such as FMEA (Failure mode effect analysis) and Ishikawa diagrams. FMEA lead to identified a failure occur in a pharmaceutical product, detectability, Risk priority number (RPN). Ishikawa diagrams segregate risks into different categories [12].
Design Space: The ICH Q8(R2) States that the design space is multidimensional combination and interaction of input variables (e.g., material attributes) and process parameters that have been demonstrated to provide assurance of quality. chance is not mean by doing work within a design. chance is considered as doing work out of design .and would begin a change in post approval process. Design space is implemented for approval and regularly assessment. It is necessary to verified a Design space at commercial scale unless it is independent. Because Design space is dependent on equipment so it may vary from lab level to industry.
Control Strategy: The capability to assess and secure the quality in-process and in final product. ICH Q8 (R2). Control strategy is defined as “a planned set of controls, derived from current product and process understanding that assures process performance and product quality”. In QbD the control strategy is established with risk assessment the criticality of the CQA is taken into consideration. The control strategy include various element such as process monitoring, characterization testing, procedural controls , in process controls , lot releasing testing , stability testing . It may incorporate with raw material attributes. environmental condition and operating systems that may impact on downstream process. e.g. Degradation is may affected by drying parameters [12].
Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing
The product Quality has been taken consideration in pharmaceutical industry. For increasing Product quality there are various tools such as PAT. The growth in manufacturing is necessary to have a Scientific knowledge. It will decrease the risk which leads to increasing in the productivity and quality. Applying the Principle of QbD to Analytical Method Which reduce the change of poor method robustness and thereby ensure that product meet its performance requirement. The Knowledge obtain during development which will support process control and design space. Thus, same method of QbD is apply to analytical and as “Analytical QbD”. AQbD consist of various tools like CQA, ATP (Analytical Target Profile) [11,12].
ATP (Analytical Target Profile)
ATP is for development process of an analytical Method. The purpose is for Design Method selection and development activities. ATP leads an improvement in Analytical method. The method is selected based on targeted analytes like API, products and impurities. To apply AQbD analytical methods has to be selected like HPLC, GC and HPTLC [11,12].
Quantitative Applications of Analytical Quality by Design
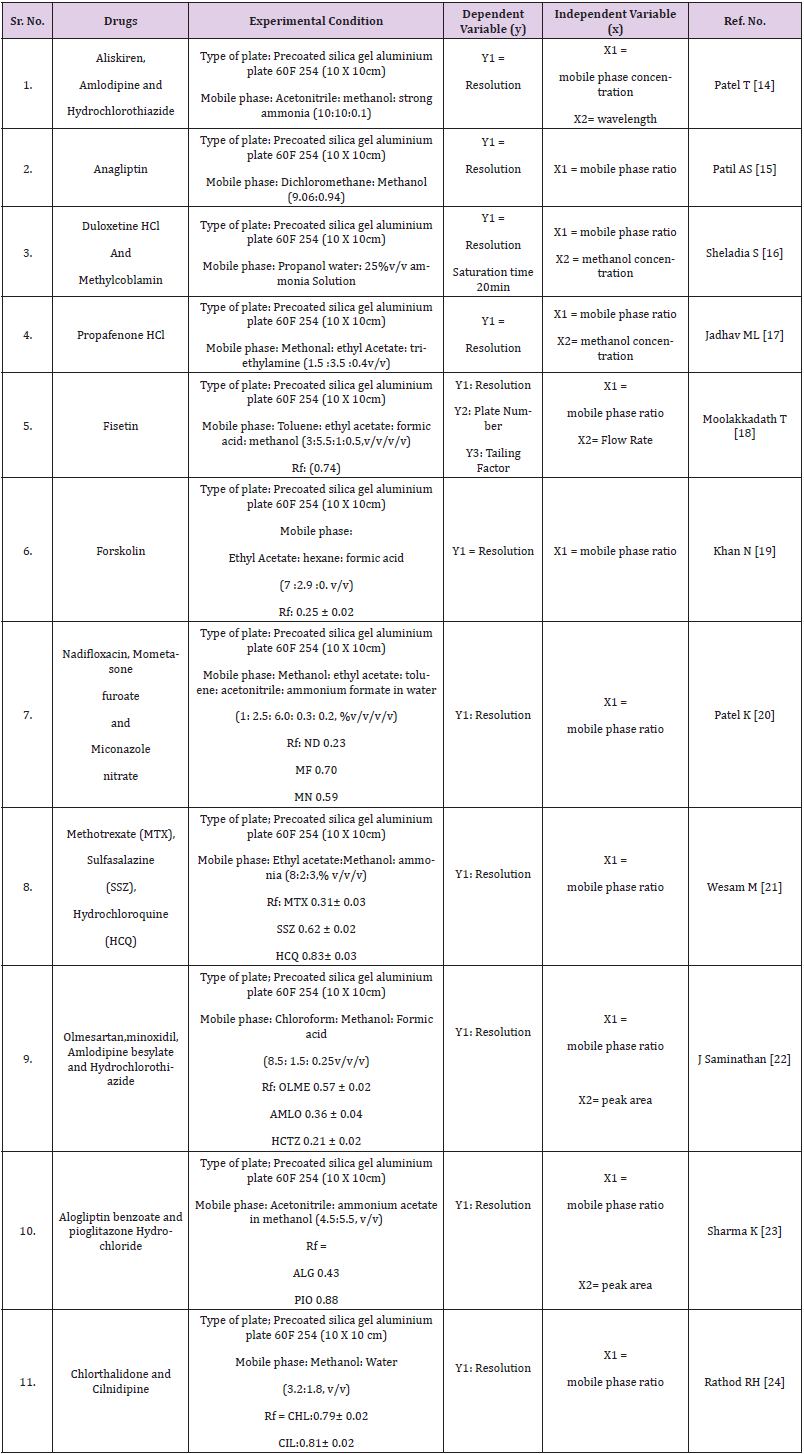
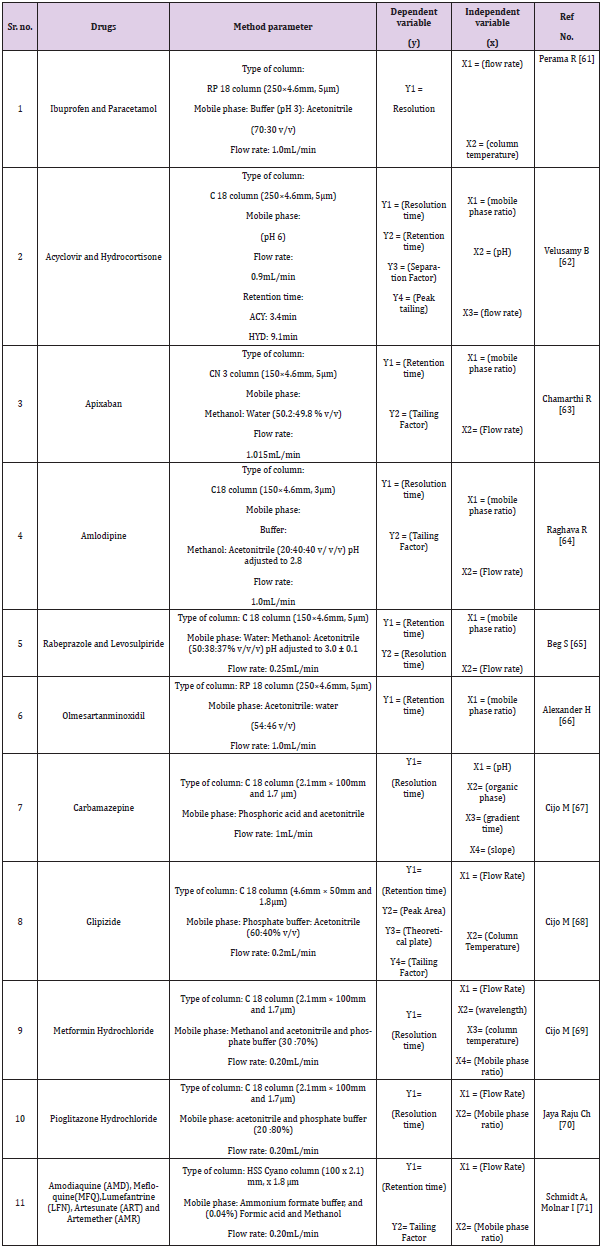
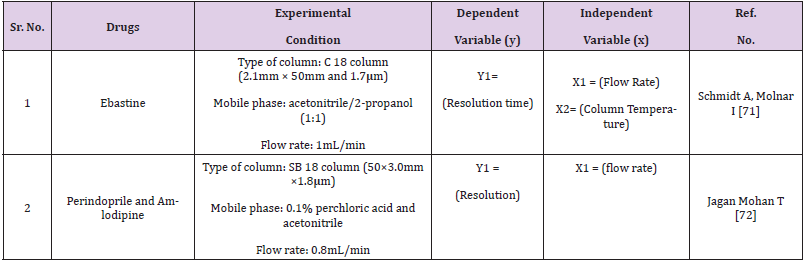
Quantitative applications by HPTLC, HPLC, UPLC and UHPLC method are given in Tables 1-4 respectively.
Result and Discussion
As we know, quality by design is an approach to implement quality in pharmaceutical but analytical QbD is a new approach to implement quality and reduce cost in pharmaceutical analysis. In this review many chromatographic methods have been covered like HPLC, UPLC, UHPLC and impurity profiling. For chromatographic methods QbD has been implemented by design expert software using different designs like central composite design (CCD), fractional factorial design (FFD), box banchan design. Gundala A [13] has developed high performance liquid chromatographic method to estimate saxagliptin (SAXA) and dapagliflozin (DAPA), to determine the essential method parameters, a risk assessment was conducted. The mathematical models were created using three independent factors: mobile phase composition, flow rate, and column temperature. The response surface methodology and the results of these independent factors were studied using a central composite design (CCD), method was optimized by mobile phase ratio, flow rate and temperature considering as an independent variable. Another study was performed to develop a new responsive and robust stability indicating HPLC method based on a fractional factorial design (FFD) approach for the simultaneous estimation of gliclazide and metformin hydrochloride in tablets without prior separation. Preliminary tests were carried out to determine the essential attribute variables, with the Taguchi screening approach being used to a large extent. two retention time (Y1) and separation factor (Y2) models were obtained and statistically interpreted. Study of constructed models and contour plots yielded the chromatographic optimum range for each input variable (Xn). The predicted data for resolution time (Y1) and separation factor (Y2) from response models were statistically important [14-72].
For the simultaneous determination of relevant organic impurities of Ibuprofen and paracetamol in a combination solid oral dosage form by reverse phase high performance liquid chromatography, a stability suggesting QbD dependent gradient method was developed and validated using the principle of consistency by design (QbD) and the design of experiments (DoE) tool (RP-HPLC). Using the “Design-Expert® 8” software tool with a quadratic mode of central composite design, the most important critical quality attributes (CQA) of the established test method were chosen and evaluated (CCD). For purity testing of ebastine and its pharmaceutical formulations, a TA stability-indicating ultra-high-performance liquid chromatographic (UHPLC) method has been developed. The robustness of the established method was investigated by varying six parameters at three levels (+1, 0, 1): gradient time, temperature, ternary composition of the eluent, flow rate, and gradient start and end concentration. The 729 experiments that resulted were carried out in silico using the previously collected data.
Conclusion
Quality by Design (QbD) is an approach to design and develop predefined product quality. QbD is a method that will improve the consistency, protection and effectiveness of products. QbD would increase the output rate; in other words, by using QbD, the risk is reduced while the input is maximised. Analytical Quality by Design applies the same concepts of QbD to analytical processes. In the pharmaceutical industry, Analytical Quality by Design (AQbD) is critical for improving product quality. It is implemented in order to reduce the number of defective products. Also, increase production. The risk can be identified early on, resulting in a highquality finished product. The various Tools are PAT, CMC, Critical Quality Attributes (CQA), Quality target Product Profile, Continuous Method Monitoring, Method optimization, and development with DOE, MODR (Method Operable Design Region), and continuous improvement. AQbD requires the right ATP and Risk Assessment of correct tools for a suitable quantity of work within proper timelines. By application of AQbd, the analytical technique makes it easier to obtain the optimum values, which makes it easier for further analysis of the drug.
The most critical process is analytical in drug production and the development of a product.
In the whole process of all the stages of the drug product life cycle, it plays a major role in development. An Analytical Method should be accurate, precise, and reliable for the intended purpose. Among all Liquid Chromatography techniques are most commonly Utilized an Overview of this article using the development of a High-performance thin-layer chromatography (HPTLC), High-performance Liquid Chromatography (HPLC), Ultra-highperformance liquid chromatography (UHPLC), reversed-phase high performance liquid chromatographic method (R-HPLC). The separation of analytes present in a sample is the key concept of an analytical method in the chromatographic method, which is developed using the QbD approach growth, which results in a highquality product. The most popular application is the assay of an active pharmaceutical ingredient (API) or determined degradation products.
For more Articles on: https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.