Celiac Disease and HLA Molecular Typing
Introduction
The History of Celiac Disease
The history of celiac disease [1] begins about 10,000 years ago when the cultivation of cereals was introduced in the area of the “Fertile Half Moon” (the strip of territory that currently includes Syria, Israel, Iran and Iraq). When this cultivation also extended to European populations, it was observed that a significant percentage of subjects did not adapt to this type of food, whose intake triggered a sort of defensive response, which could evolve into atrophy of the jejunal mucosa. Throughout history, many doctors have been involved in studying the symptoms that occurred following the intake of wheat. The first doctor who reported celiac disease in adults was Areteo of Cappadocia in the 1st century AD calling it “celiac diathesis”, a term that literally means “intestinal alteration”. In 1888 Samuel Jones Gee was the first doctor to report celiac disease in children, describing in great detail the picture of the typical form and realizing that the cause of celiac disease was to be sought in a food, although he could not identify that responsible. In 1889 Gibbons pointed out that this disease affected children and called it “the celiac affection in children”, claiming that toxic substances absorbed from undigested food were harmful to the child’s body. In 1903 V.B. Cheadle called celiac disease “acholia” because the stools emitted by patients were very light in color due to the presence of lipids.
In 1908 G.A. Herter introduced a new concept of celiac disease, he thought it was due to inflammation of the intestine caused by the excessive development of the intestinal flora (intestinal germs) and he called it “infantilism from chronic intestinal infection”. However, a greater interest in celiac disease began after 1945 when the pediatrician Willem-Karel Dicke of the Children’s Hospital in Utrecht, identified wheat flour as the cause of the disease. The criteria for formulating a diagnosis took a long time to be established, as initially celiac disease could be hypothesized and subsequently verified only after death by carrying out an autopsy and examining the intestinal mucosa. With technological progress it has been possible to remove the duodenal and jejunal mucosa even in the subject still alive and then the criteria to be followed for the diagnosis of celiac disease have also been established. Up to 1988, three fasting biopsies were required to diagnose celiac disease. At present, celiac disease is a disorder of great scientific interest and is defined as an autoimmune disease primarily localized in the small intestine (autoimmune enteropathy), triggered by the ingestion of gluten-containing flours by genetically predisposed subjects [2].
The HLA System
The HLA (Human Leukocyte Antigens) system includes a group of genes and their products that play a fundamental role in the acquired immune response; these molecules in humans encode cell-surface proteins responsible for the regulation of the immune system [3]. The genes of HLA system are located on the short arm of chromosome 6 (6p21) and are divided into two main classes: class I (MHC-I) which includes 3 loci A, B, C and class II (MHC-II or HLAD), which includes the genes DP, DQ, DR [4,5]. Class I antigens are found on the surface of all nucleated cells, while class II antigens are found on antigen presenting cells (APC), macrophages, and T and B lymphocytes [6-8]. HLA genes express proteins with a tetrameric structure similar to immunoglobulins structure, with a molecular weight of about 150 kDa. These proteins are heterodimers of two noncovalently associated glycosylated polypeptide chains (α and β chain). An extracellular portion composed of two domains (α1 and α2, or β1 and β2) is anchored on the cellular membrane through a short transmembrane region. Among their many functions, HLA molecules play a fundamental role in the recognition and response to transplantation of solid organs or hematopoietic stem cells [9]. The first scientific papers on the association of HLA molecules with certain diseases were published about 50 years ago [10,11]. The HLA region, with over 200 gene loci distributed in 3.6 Mb, is one of the regions with the highest gene density of the entire human genome. An important feature is that and a lot of genes in this region are highly polymorphic. Classical HLA class I and II genes have been associated with more than 100 diseases [12-14].
HLA and Celiac Disease Association
The significant peculiarity of celiac disease, compared to other autoimmune diseases, is undoubtedly the knowledge that the gliadin protein of the grain of wheat is an environmental trigger. The key activators of celiac disease are the specific immunogenic peptides of gliadin (rich in glutamine and proline) which are able to reach the basal lamina of intestinal cells thanks to modifications of the intercellular tight junctions and the increase of intestinal permeability that occurs in this pathology as well as in other gastrointestinal inflammatory diseases. This condition promotes the involvement of tissue type II transglutaminase (tTG) (protein glutamyl γ-glutamyltransferase, TG2-EC 2·3.2·13), an ubiquitous calcium-dependent intracellular enzyme that is thought to act as a deamidase converting glutamine residues to glutamic acid and generating specific negatively charged gliadin peptides that bind with high affinity to coeliac-associated HLA-DQ2 or DQ8 molecules [15,16]. The peptides thus modified are presented in complexes with HLA DQ2 or DQ8 of antigen-presenting cells (dendritic cells, macrophages, B cells) to CD4+ T lymphocytes. Recognition of the peptide-HLA complex by T lymphocytes is responsible for their activation and the release of multiple cytokines. Some cytokines promote the activation and clonal expansion of B cells which produce antibodies against gluten but also autoantibodies against tTG; other cytokines have a pro-inflammatory action and stimulate the secretion of matrix metal proteases by fibroblasts and inflammatory cells with consequent tissue remodeling [17,18].
Tissue damage results in a further release of tTG in the extracellular compartment. As a consequence of the increase in the CD4+T lymphocyte infiltrate in the lamina propria, there is an increase in the intraepithelial lymphocyte infiltrate (CD4+ and CD8+) which determines the destruction of the epithelium through their cytolytic activity. The lesions of the intestinal mucosa (atrophy of the villi and hyperplasia of the crypts) are the result of this dynamic and time-modulating immunological process [19]. The genetic component of celiac disease is strongly suggested by numerous evidences such as the increased risk of disease in firstdegree relatives [20]. Infact, HLA-DQ2 and HLA-DQ8 molecules play a fundamental role in the pathogenesis of numerous autoimmune disorders. These are αβ heterodimers, consisting precisely of the union of an α chain and a β chain, of which there are numerous allelic variants and whose expression occurs in part from HLADQA1 (α) and HLA-DQB1 (β) genes. The presence of allelic variants capable of encoding for the heterodimers DQ2 and DQ8 predisposes to celiac disease.
HLA-DQ2 and DQ8 Molecules
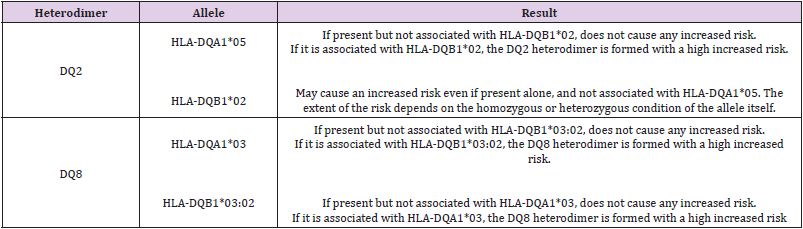
The presence of DQ2 (complete heterodimer or only DQB1* 02 allele) and/or DQ8 causes an increase in the risk of celiac disease, depending on the different combinations, up to about 14 times that of the general population, while the absence of the same factors makes the development of the disease quite unlikely. DQ2 and DQ8 are glycoproteins present on the surface of some cells of the immune system consisting of two different chains (heterodimers) α and β [21]. The α and β chains are encoded by the DQA1 and DQB1 genes respectively. The alleles DQA1*05 and DQB1*02 encode for the heterodimer DQ2 associated with higher risk of celiac disease while the alleles DQA1*03 and DQB1*03:02 encode for the heterodimer DQ8 associated with a lower risk of celiac disease. Among celiac patients, more than 80% is DQ2 (DQA1*05 and DQB1*02), 10% is DQ8, 5% is DQB1*02 positive but DQA1*05 negative, carrying only the β chain of the DQ2 molecule. Rare cases have none of these combinations, giving an important negative predictive significance to the test [22]. Homozygous DQB1*02/*02 status determines the highest risk of celiac disease and has been reported in association with more severe forms with complications such as dietary refractoriness and development of lymphomas [23].
Which HLA Alleles to Test
Typing performed with molecular biology techniques should include: the alleles DQA1*03, DQA1* 05, DQB1*02 and DQB1*03: 02 (Table 1). There is a gradient of risk: homozygous DQB1*02 state > DQ2 heterodimer > DQ8 > presence of only the DQ2 β chain). Using PCR-based techniques is possible to examine the HLA profile in high resolution for the DQ2 and DQ8 alleles involved in the disease.
Conclusion
Celiac disease is an autoimmune enteropathy triggered by the ingestion of gluten-containing flours in genetically predisposed individuals. It occurs in the small intestine and can have different symptoms: weight loss, dystrophy, growth failure, lack of vitamins and microelements, iron deficiency anemia, osteopenia/ osteoporosis, meteorism and the presence of fat in the stool. Gluten is deaminated by transglutaminase and recognized as “non-self” by specific HLA alleles in an immunological reaction in which T cells of celiac individuals recognize gliadin fragments only if these are presented by antigen-presenting cells carrying the HLA-DQ2 or DQ8 alleles. HLA molecular typing in celiac disease is a molecular test that evaluates the predisposition of an individual to develop the disease based on the presence/absence of genetic risk factors (DQ2, DQ8). The presence of one of the predisposing HLA combinations determines an increased risk of celiac disease, while the absence of HLA-DQ2 or DQ8 makes development completely unlikely. Genetic testing should be used primarily for its predictive significance since subjects negative for DQ2 and DQ8 will never exhibit the autoimmune reaction triggered by gluten. HLA typing has a welldefined role in the diagnostic process of celiac disease. The correct use of this analysis is of great importance both for the economic implications related to the diagnosis of celiac disease and for the follow-up of genetically susceptible patients and family members. Ultimately, this molecular investigation proves to be a valid help for the clinician in cases of diagnostic uncertainty.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.