Treatment of Epstein-Barr Virus, Chronic Fatigue, Mitochondrial Dysfunction, and Insomnia
Introduction
Epstein-Barr virus (EBV) is ubiquitous in adults (90-95%) and is a DNA, γ-herpes virus, also known as Herpes Simplex Virus 5, HSV- 5, or Human Herpesvirus 4, HHV-4). EBV is associated with chronic fatigue syndrome (CFS) (Blomberg, et al. [2-10]). CFS symptoms include persistent fatigue that is not due to ongoing exertion, not alleviated by rest, and has reduced the patient’s activity level substantially. (White, et al. [9]) notes that two post-infectious-EBV forms exist:
(1) With insomnia and
(2) With hypersomnia.
(Cooke, et al. [3]) associate latent EBV infection with psychiatric disorders (e.g., depression and manic-depressive illness). An EBV infection typically begins with a severe, acute flu-like infection: severe lethargy/fatigue, fever, and fever-induced sleep without GI upset. This acute phase is usually called infectious mononucleosis. (McFadden, et al [11]) found that EBV initially induces hyperproliferation that is quickly suppressed by activation of DNA damage response and G1/S-phase growth arrest with a rapid recovery (<72 hours) in immuno-competent patients. Recovery may take 6 months or more in immune-compromised patients. The typical virus size is 0.02-0.4 microns, allowing viral penetration of a human-cell wall (Subramanian, et al. [12]). Inside the cell, the viral genome is injected into the cell’s cytoplasm, hidden from the immune system, while the virus replicates and gains control of cellular functions after the acute infection. This latent infection causes metabolic hijacking (Lange, et al. [13-16]) of acetyl-CoA, acetylation, glycolysis, Krebs cycle, lipid synthesis, and EBV-infected immune cells [B-cells (Hulse, et al. [16-18]); T- and natural-killer-cells (Imashuku [19]). The latent EBV infection normally continues for the rest of the patient’s life. The arrested cells have a reduced level of mitochondrial respiration and a decrease in gene expression for the Krebs cycle and oxidative phosphorylation. Arrested cells are characterized by increased expression of p53 pathway gene targets, including sestrins leading to activation of AMPK, reduction in mTOR signaling, and increased autophagy. A concomitant increase in glucose imports and surface glucose transporter 1 (GLUT1) occurs, leading to elevated glycolysis, oxidative phosphorylation, and suppression of basal autophagy. (Xiao, et al. [20, 21]) found that latently infected cells have enhanced glucose and glutamine uptake with deregulated glycolysis. (Li, et al. [18]) showed that fatty acid synthesis is important for EBV lytic replication. The EBV latent membrane protein 1 (LMP-1) is expressed in many (but not all types) of EBV-1 and can induce SREBP1 and FASN, leading to increased lipid droplets (Lo, et al. [44]), sometimes leading to slightly elevated LDL.
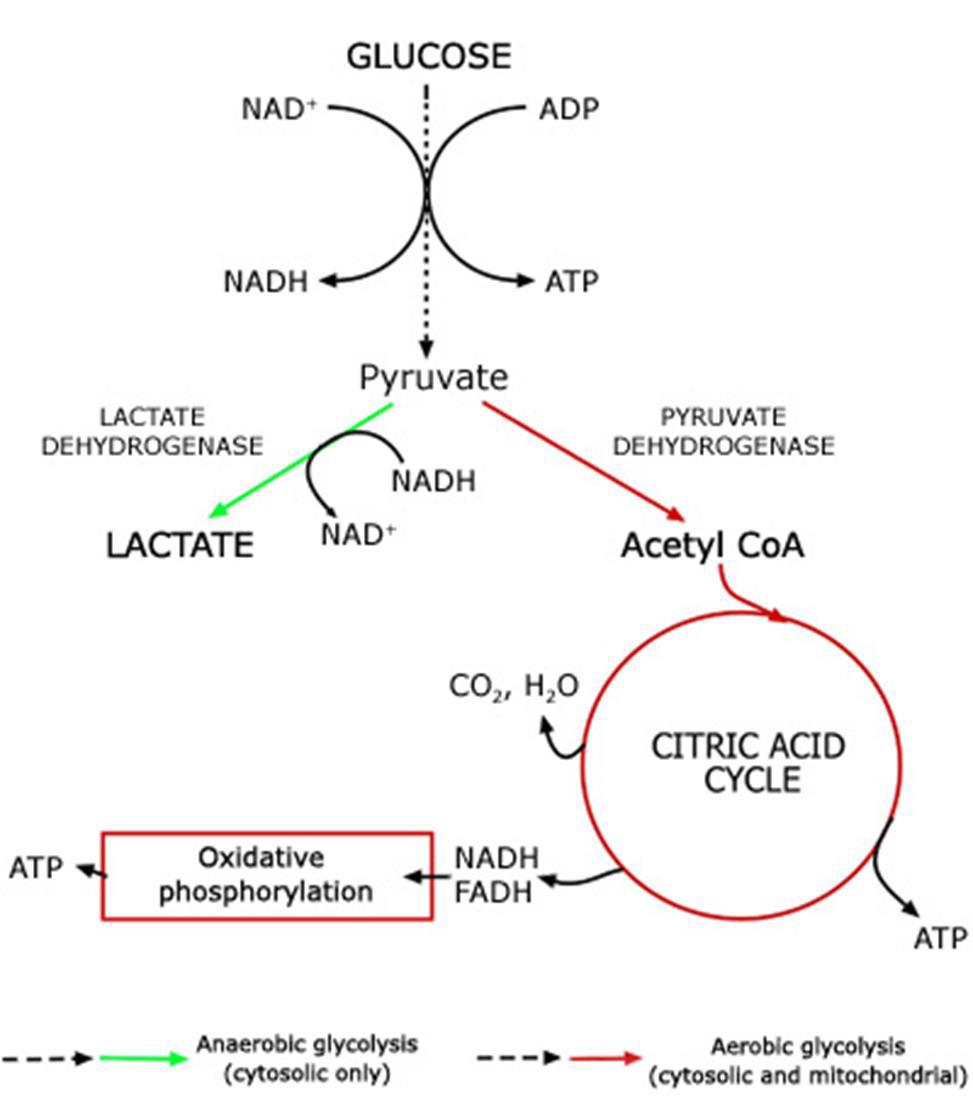
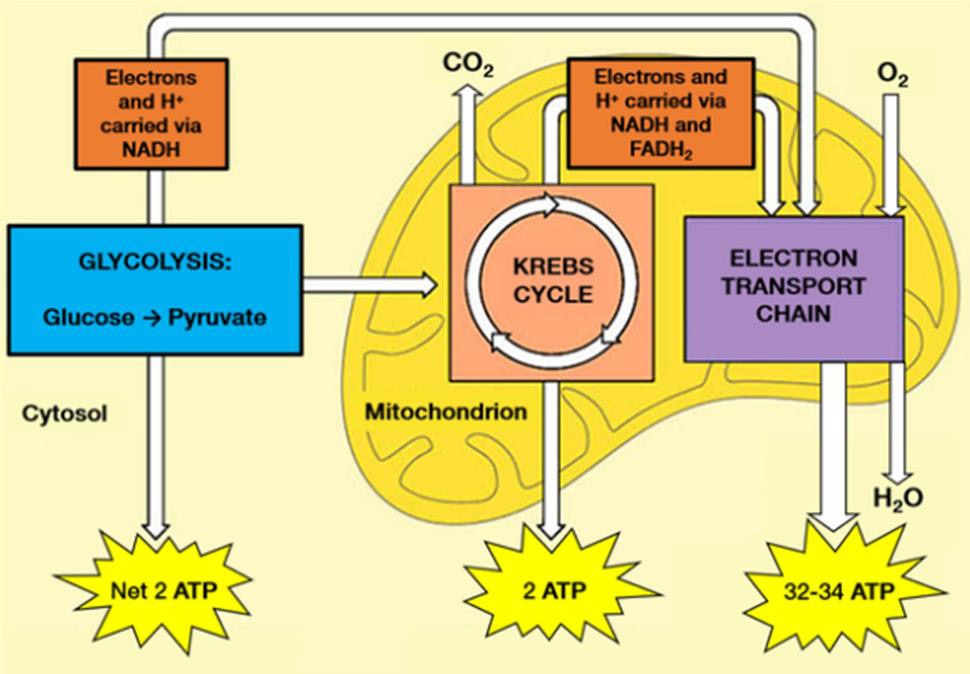
Adenosine triphosphate (ATP) is the body’s currency of energy transfer. Figure 1 shows metabolism beginning with glycolysis that processes glycose into pyruvate, ATP, and lactic acid. Lactic acid (lactate) is a waste product that is converted via the liver’s Cori cycle into glucose; this conversion is particularly efficient in highly trained athletes [McArdle, et al. [22,23]). Pyruvate enters the Krebs’ cycle, which produces more ATP (Figure 2). Excess electrons and H+ from the Krebs’ cycle are converted into much more ATP in the electron transport chain (ETC). The Krebs’ cycle and ETC occur inside mitochondria. A typical cell has hundreds of mitochondria, while ~2000 are present in liver cells and none occur in mature red blood cells. Metabolic hijacking involves mitochondrial dysfunction.
Figure 1: Conversion of glucose into ATP, lactate and pyruvate that then enters the Krebs’ cycle.
Note: https://acutecaretesting.org/en/articles/lactate-and-lactic-acidosis/; accessed 12Sep2021
Figure 2: Glycolysis processes glucose from food into pyruvate, plus two molecules of ATP. Pyruvate then enters the Krebs’ cycle (also called the citric acid cycle or tricarboxylic acid cycle) and is converted into two more ATP molecules. Excess electrons (carried by electron donors) and excess protons (H+) from the Krebs’ cycle are metabolized into many more ATP molecules in the electron transport chain (ETC). See [A.L. Lehninger et al. [43] for details.
Treatment of Mitochondrial Dysfunction
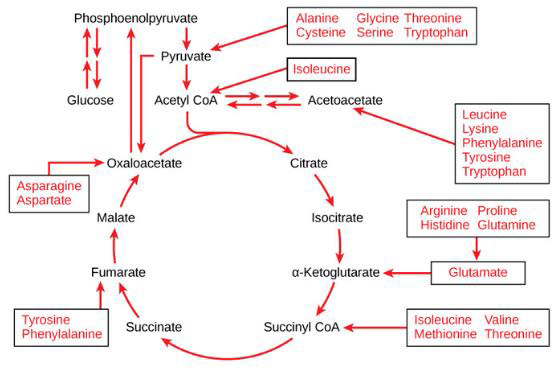
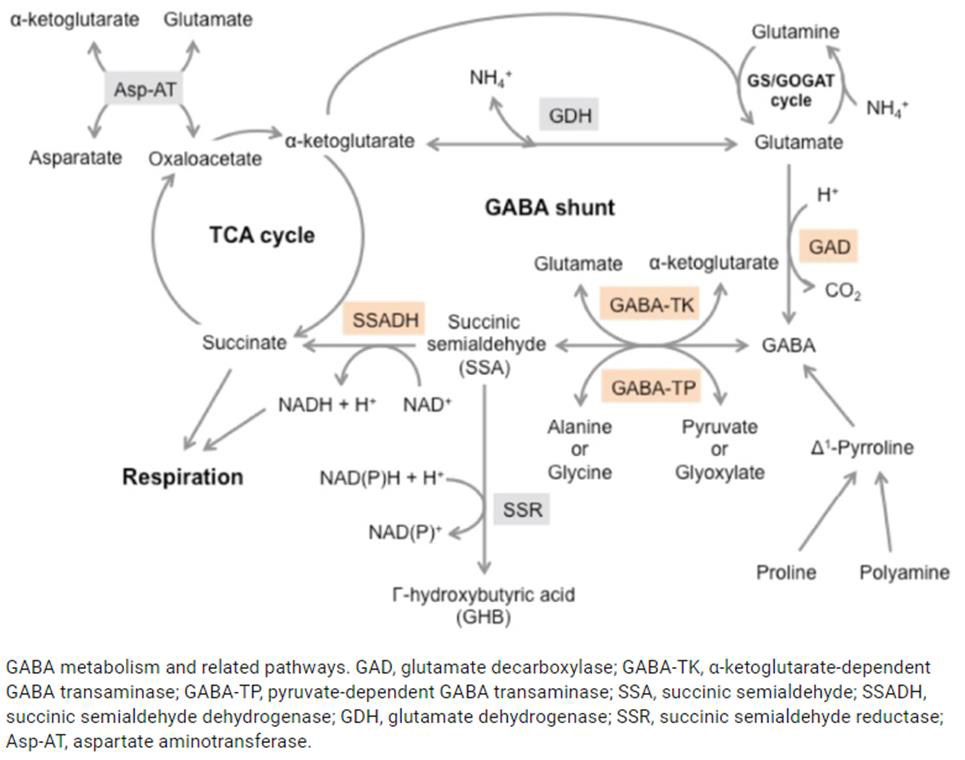
Mitochondrial dysfunction impairs ATP production, causing the diseases, as noted above. Amino and fatty acids from food can enter the Krebs’ cycle (Figure 3). Thus, supplementation with freeform amino acids can boost energy and mitigate hypoglycemia under stress conditions (e.g., trauma, exercise, starvation, and disease) (Dohm, et al. [24-26]). The GABA shunt diverts GABA (gamma-aminobutyric acid) from the production of GHB (gamma hydroxybuterate) into production of succinate (Figure 4) under stress conditions (e.g., latent EBV infection that causes insomnia due to GABA and GHB deficiencies. Mitochondrial dysfunction can also arise from cofactor deficiency, for which vitamin and mineral supplementation is appropriate (DiPasquale [27]). Many co-factors are helpful in treating insomnia [e.g., B1 (250 mg), B2, B3 (250 mg), B5 (500 mg), B6 (25 mg, as P5P), B9 (as methyl folate—680 mg), B12, vitamin C, calcium citrate (315 mg), GABA (300 mg), Mg (1.2 g), Zn, lipoic acid, tryptophan (1 g), fish oil (1 g)]. These co-factor deficiencies cause melatonin deficiency (Figure 5) and deficiencies in GABA and GHB (Figure 6). The commonalities in these pathways are magnesium, B6, zinc, and copper (an inhibitor to avoid after noon). B6 supplementation markedly improves sleep, along with a GABA-agonist (e.g., 1 mg Clonazepam). A review on mitochondrial dysfunction proposes antioxidants (CoQ10, alpha lipoic acid, and acetyl-L-cysteine) that are useful in singleton and paired combinations, but never tested as a triad (Pagano, et al. [28]). Other antioxidants are glutathione (poorly absorbed orally) and N-acetylcysteine (precursor of glutathione). Anti-inflammatories include turmeric (450 mg) and vitamin D (5000 IU).
Note: https://courses.lumenlearning.com/boundless-biology/chapter/connections-of-carbohydrate-protein-and-lipidmetabolic- pathways/
Figure 3: Proteins are converted into amino acids by a variety of enzymes. Usually, amino acids are recycled into the synthesis of new proteins or are used as precursors for synthesis of other important biological molecules (e.g., hormones, nucleotides, neurotransmitters). A deficiency in Krebs’ cycle reactant can cause shunting of some amino acids into the Krebs’ cycle, as shown here.
Figure 4: The GABA shunt bypasses two steps (oxidation of α-ketoglutarate to succinate) of the tricarboxylic acid (TCA) cycle via reactions catalyzed by three enzymes: glutamate decarboxylase, GABA transaminase, and succinic semialdehyde dehydrogenase. The GABA shunt plays a major role in primary carbon and nitrogen metabolism and is an integral part of the TCA cycle under stress conditions [Takayama and Ezura [45]].
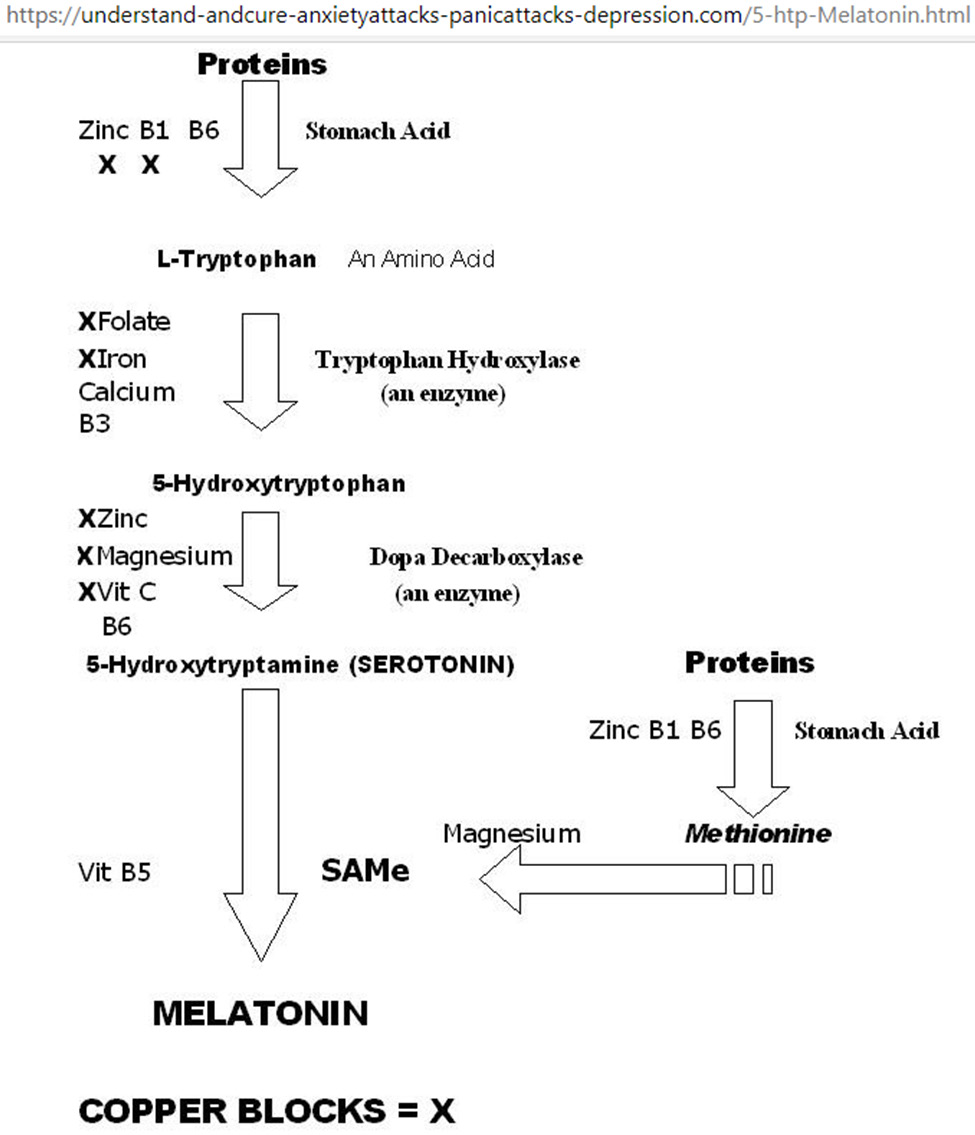
Figure 5: Metabolic network for tryptophan-5HTP-setotonin-melatonin. Starting from the top, vitamins B1/B6 and zinc are required for stomach acid production to break down proteins into amino acids; excess copper blocks B1 and zinc as co-factors for stomach acid production. Serotonin promotes happiness; serotonin deficiency results in depression, and/or anxiety, and/ or panic attacks. Melatonin promotes sleep, melatonin deficiency results in insomnia. Supplementation with Mg, Ca, Zn, and vitamins B1, B3, B5, B6, B9, and C improves sleep quality.
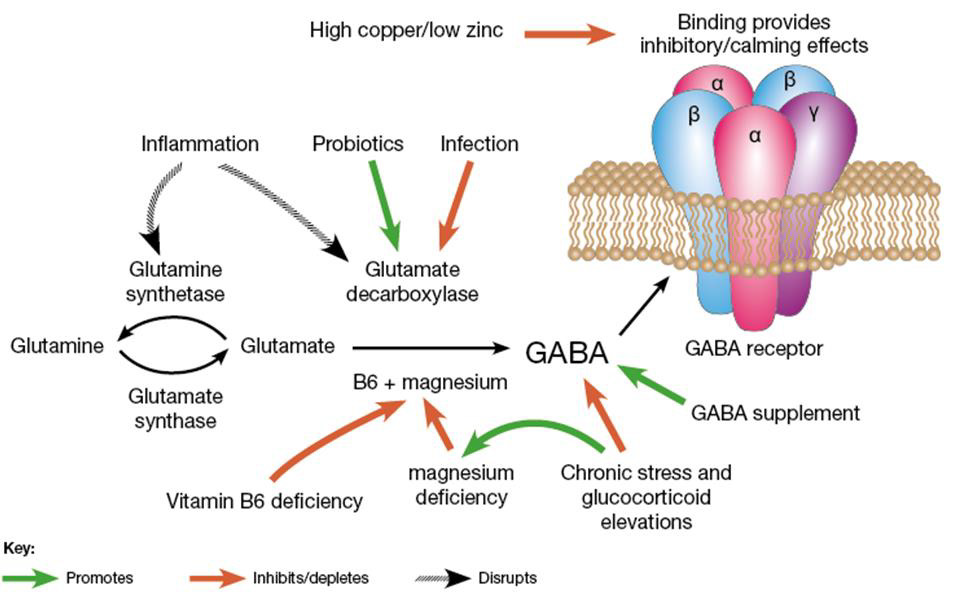
Figure 6: Metabolic network for glutamine-glutamate-GABA. Excess Cu inhibits this network as in Figure 5. B6, Mg, Zn are also co-factors in this network. [(https://drjockers.com/gaba/) accessed 15May2021].
High-GABA food (e.g., tomatoes, spinach, oats, potatoes, sweet potatoes, corn) improves sleep. High-glutamate food (e.g., tomatoes, eggs, cheese, salmon, beef, spinach, nut butter, Parmesan cheese) is converted to GABA via B6 and magnesium for sustained sleep. Whole wheat bread (2 slices at supper) facilitates tryptophan transport across the blood-brain barrier to improve sleep. Latent EBV infection activates immune response of interferon-gamma (IFN- γ) that in turn triggers indoleamine-2,3-dioxygenase (IDO), which metabolizes tryptophan to kynurenine (rather than to 5HTP→serotonin→melatonin (Mehraj, et al. [29,30]). EBV’s diversion of tryptophan to kynurenine decreases serotonin, and thus increases depression (Anderson, et al. [31]), making 5HTP a potential alternative to anti-depressive medications. (Schwarcz [32]) found that metabolites of the kynurenine pathway influence glutamatergic activity, including ionotropic and metabotropic receptors, vesicular glutamate transport, and generation/ scavenging reactive free radicals.
(Kermani [5]) notes that chronic (latent) EBV infection is associated with
a) Over-burdened organs, including the liver, pancreas, spleen, and gut
b) Cardiac dysrhythmias,
c) Elevation of IgG, IgM-antibodies and anti-nuclear body,
d) Reduction in natural killer and lymphocyte helper cells,
e) Deficiencies in trace elements, vitamins, antioxidant capacity,
f) Excess of heavy metals,
g) Hashimoto’s thyroiditis, which is usually associated with heavy metal toxicity, e.g., amalgam fillings,
h) Dental infections,
i) Additional lymphotropic pathogens, such as cytomegaloviruses,
chlamydia, toxoplasma, and borrelia.
Treatment recommendations by (Kermani [5]) include alkaline infusion, vitamins B6/B9/B12/C, magnesium, selenium, zinc, and algae.
Numerous antivirals are used to treat EBV (Lieberman [6]), including:
• Glycyrrhizin in licorice root (Lin, et al. [33])
• Lauric acid/monolaurin,
• Butyrate in the form of tributyrin (Szentirmai [34])
• Quercetin
• N-acetyl cysteine,
• Co-enzyme Q-10,
• Exercise,
• Purified protein A from calf thymus (Riordan, et al. [35]),
• Proteflazid [Chopyak, et al. 2008],
• Valtrex (Sunde [36]).
Acknowledgment
Enlightening discussions with Rachel A. Wilkenson MD motivated this work, which was unfunded.
Conclusion
Ongoing research seeks novel diagnostics and therapeutics for EBV/CFS and EBV-induced cancers, such as EBV nuclear antigen 1 and EBV-oncoproteins, LMP1 and LMP2A (Andrei, et al. [37-48]).
For more Articles on: https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.