Types and Treatments of Leishmaniasis
Introduction
The Leishmaniasis are a cluster of parasitic diseases produced by morphologically alike parasites in the genus Leishmania with Order Kinetoplastida and Family Trypanosomatida. The disease is transferred through the bite of infected Phlebotomine Sandfly, which becomes infected by taking blood meal from infected mammalian host. A total of about 30 species in Phlebotomus genus and Lutzomyia genus have been recognized as vectors (Desjeux P [1]). Sandflies are comparatively weak, silent flyers; they rest in dark, moist places, and are usually most active in evening and at night-time hours. Adventure travelers, bird spectators, priests, army employees, building workers, and researchers on night time tasks are at higher risk of being exposed to sandflies. The clinical spectrum of leishmaniasis ranges from a self-resolving cutaneous ulcer to a mutilating mucocutaneous disease and even to a lethal systemic illness. Therapy has long been a challenge in the more severe forms of the disease, and it is made more difficult by the emergence of drug resistance. With the exception of Australia, the Pacific Islands, and Antarctica, the parasites have been identified throughout large portions of the world. The WHO (world health organization) has rated leishmaniasis as the sixth largest infective disease (WHO [2]).
Epidemiology of Leishmaniasis in the World
Recurrent epidemics of visceral leishmaniasis in East Africa (Ethiopia, Kenya, South Sudan and Sudan) have caused high morbidity and mortality in affected communities. Likewise, major epidemics of cutaneous leishmaniasis have affected different parts Afghanistan and the Syrian Arab Republic. In 2017, 20 792 out of 22 145 (94%) new cases reported to WHO occurred in seven countries: Brazil, Ethiopia, India, Kenya, Somalia, South Sudan and Sudan. In the WHO South-East Asia Region, the kala-azar elimination programme is progressing satisfactorily, and countries such as Bangladesh that reported more than 9000 cases in 2006 reported 255 and 192 new cases in 2016 and 2017, respectively. The majority of cutaneous Leishmaniasis cases occur in Afghanistan, Algeria, Brazil, Colombia, the Islamic Republic of Iran, Pakistan, Peru, Saudi Arabia and the Syrian Arab Republic. Anthroponotic cutaneous leishmaniasis (where humans are the major reservoir of the parasite) is predominantly urban and periurban, and shows patterns of spatial clustering similar to those of anthroponotic visceral leishmaniasis in South-East Asia. The disease is usually characterized by large outbreaks in densely populated cities, especially in war and conflicts zones, refugee camps and in settings where there are large-scale migration of populations. The epidemiology of cutaneous leishmaniasis in the Region of the Americas is complex, with intra- and inter-specific variation in transmission cycles, reservoir hosts, sandfly vectors, clinical manifestations and response to therapy, and multiple circulating Leishmania species in the same geographical area. Almost 90% of mucocutaneous leishmaniasis cases occurs in the Plurinational State of Bolivia, Brazil and Peru (WHO [2]).
Clinical Forms of Leishmaniasis
Leishmaniasis currently threatens 350 million men, women and children in 88 countries around the world. The leishmaniasis are parasitic diseases with a wide range of clinical symptoms:
1) Cutaneous Leishmaniasis is the most common form. The parasite species are divided into old world (Southern Europe, the Middle East, Asia and Africa): Leishmania tropica, leishmania major, and leishmania aethiopica and new world leishmaniasis (Latin America): leishmania mexicana and leishmania braziliensis as reported by Umi Azizah and Yadhu Nurdian. Cutaneous forms of the disease normally produce skin ulcers on the exposed parts of the body such as the face, arms and legs. The disease can produce a large number of lesions sometimes up to 200 causing serious disability and invariably leaving the patient permanently scarred, a stigma which can cause serious social prejudice. The incubation period of this ranges from 2 to 6 weeks approximately.
2) Diffused cutaneous leishmaniasis is very rare even in countries where leishmania is endemic. It is caused by L.mexicana and L.aethiopica as reported by Consuelo V. David and Noah craft. Disease begins with a primary, painless small pimple at the spot of inoculation and proceeding to diffuse, non-ulcerating, erythematous to violet color macules, nodules, and plaques excessively infiltrated with amastigotes. The face, upper and lower extremities, and buttocks are most affected (David, et al. [3]).
3) Mucocutaneous leishmaniasis is a life threatening in contrast to cutaneous leishmaniasis and requires treatment. This disease is caused by leishmania species of viannia subgenus leishmania viannia braziliensis, leishmania viannia amazonesis, leishmania viannia panamensis, and leishmania viannia guyanesis. Clinical progress of this disease depends on the collaboration of host cell-mediated immunity and parasite virulence. The patient has scars from the prior occurrence of CL. Early ML starts with the erythema and ulceration of the nostril. After that, there is continuous destruction of the cartilaginous facial and upper airway structure, and oronasopharyngeal mucosa, following in secondary infection, disfiguration, and airway obstruction. Indication of intracellular amastigotes and biopsy are needed for diagnosis (Ahluwalia, et al. [4]).
4) Visceral leishmaniasis is caused by the species Leishmania Donovani. Post-kala-azar dermal leishmaniasis is the dermal action of VL. PKDL ranges from hypopigmented macules to infiltrated papules. Depending on the species and geographic area, the infection may or may not be developed. PKDL lesions may serves as a reservoir of parasites. Diagnosis is focused on trends of epidemiology and clinics. The gold standard diagnoses are the culture of the tissue and slit smear.
Morphology of Leishmania
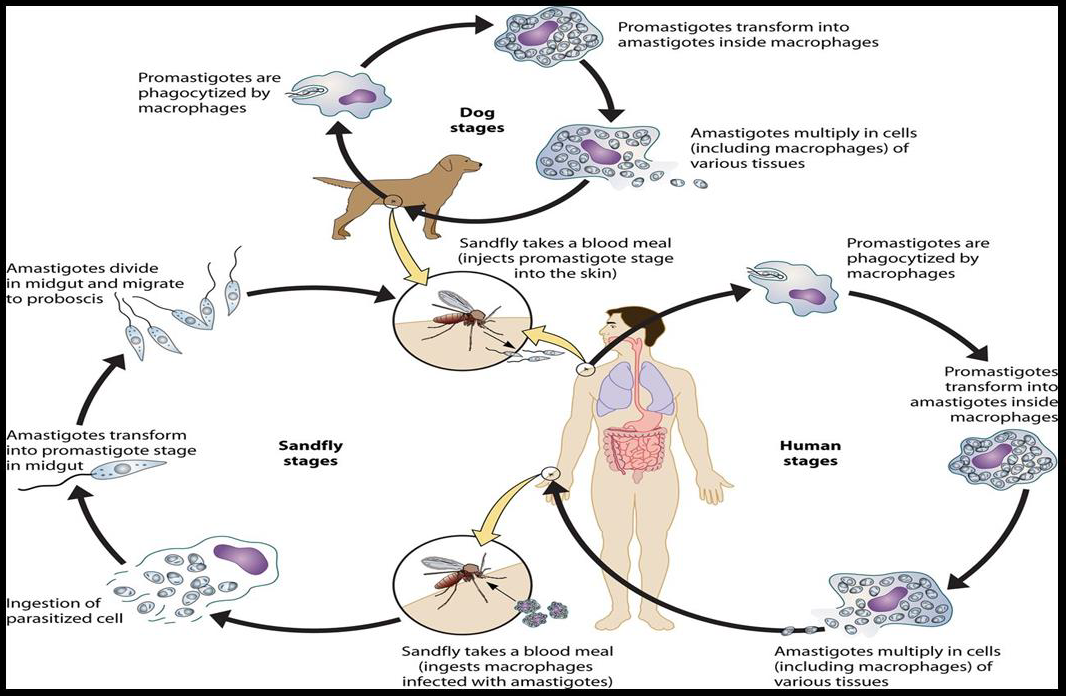
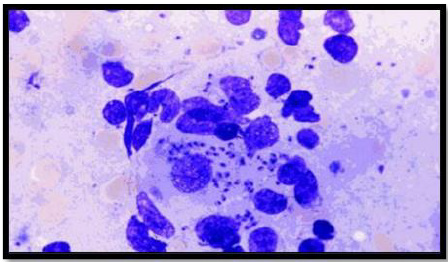
Leishmania has two forms, the amastigote and the promastigote. Amastigote forms are found in the vertebrate host whereas promastigote forms are found in the vector (Unat, et al. [5-7]). During feeding, sandflies inject infectious promastigotes into a susceptible mammal. Promastigotes are phagocytes, converted into amastigotes in the tissue level, and multiplied by simple division within these cells. The parasite continues to infect phagocytic cells either at the skin infection site or in secondary lymphoid organs. Sandflies are infected with an active skin lesion in CL or parasitemia in VL by feeding on the host. In the sandfly midgut, parasites transform to promastigotes. Promastigotes migrate from the midgut and become heavily infected (Esch and Petersen 2013). The life cycle of Leishmania is shown in Figure 1. Amastigotes, shown in Figure 2, are 2-4m in length, are ovoid or round in shape. In addition, they are usually found in monocytes, polymorphonuclear leukocytes and endothelial cells. When stained with Giemsa, the cytoplasm appears in blue and nucleus in pink or dark red, respectively (Unat, et al. [5,7]). Kinetoplast is rod shaped and stained in dark red, shiny red or purple. Amastigotes are nonmotile, feed on via osmosis and get nutrient from tissues. They are aerobes and proliferate longitudinal by binary fusion in macrophages. Firstly, Kinetoplast and blepharoplast and then nucleus and cytoplasm are divided. There is a large nucleus close to the cytoplasm and the Kinetoplast adjacent to the nucleus (Unat, et al. [5,7]). Additionally, there are vacuoles, blepharoplast and axonem in the cytoplasm. Flagellum does not come out of the cell freely. In all Leishmania species, there is only one mitochondrion, the Golgi apparatus and lysosome that helps to feed on parasites by various enzyme activities (Unat, et al. [5,7]).
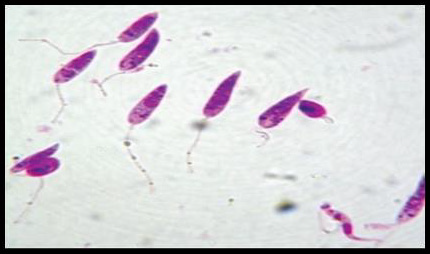
Promastigotes, shown in Figure 3, are 15-28m in length and 1.5- 3.5m in width. One end is sharper and the other end is ovoid. Flagella come up from the front edge. As a result of dying with Giemsa, cytoplasm is stained in blue, inside the cytoplasm nucleus is stained in pink or red (Ozbel, et al. [7]). Kinetoplast is stained in lilac or shiny red in front of the nucleus. Blepharoplast is present before Kinetoplast. Promastigotes are found in the midgut of the vector and the culture medium when amastigotes develop into promastigotes. There are free flagella and axonem which is located near to blepharoplast (Ozbel, et al. [7]). Additionally, kinetoplast, nucleus, nucleolus and pores located in nucleus membrane are present. Moreover, the Golgi apparatus and the endoplasmic reticulum are found in the cytoplasm (Unat, et al. [5,7]).
Leishmanolysin (gp63)
Leishmania species express a membrane glycoprotein on their surface which is called leishmanolysin. The Leishmania ‘s major surface glycoprotein, referred to as gp63, is a 63k Dalton zinc metalloproteinase containing a glycosyl phosphatidyl inositole (GPI) membrane, the major component of promastigotes (5x105 molecules / cell), but at a lower surface density (Casgrain, et al.). It has been stated by Elfaki, et al. [8] that gp63 mainly act in receptor mediated uptake of the promastigotes in the mammalian host by macrophages (Elfaki, et al. [8]). Because of its abundance, surface location and proteolytic activity, the importance of the gp63 in binding promastigotes to macrophages has been inferred. It is one of the parasite receptors that is reported to enhance phagocytosis and increases the survival of extracellular promastigote stage in the presence of host complement and promotes amastigote survival within macrophage phagolysosomes (Chang, et al.). Gp63 prevents parasite from lysosomal cytolysis, exert control over complement activation and degradative activities of macrophages by its protease activity (Alexander, et al. [9]). With the aid of macrophages it will protect the liposome-encapsulated proteins from phagolysosomal degradation. Therefore, gp63 is primarily active in binding macrophage to the Leishmania, in replication and in intramacrophage survival (Razzazan, et al. [10]). It has been suggested that gp63 may interact directly with macrophage receptors, including receptor type 3 macrophage complement, and receptor fibronectin (Rizvi, et al.). This cleaves a number of substrates found on human T-cells, including the CD4 differentiation molecules cluster.
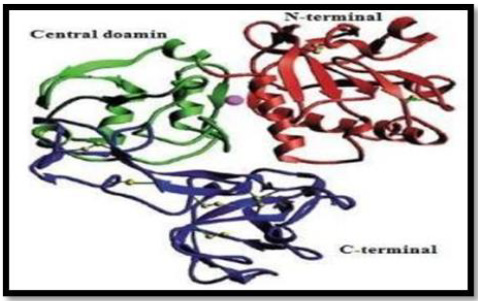
In addition, Lieke et al, noted that gp63 influences proliferation and interferon-gamma unconfined by natural killer cells (NKs) in humans, thus limiting immune response (Lieke, et al.). It has many features that mimic matrix members such as fibrinogen, Zn2 + requirement, cell surface presence, and the sequence resemblance of the proposed active site (Button, et al.). In 1998, the crystal structure of L.major gp63, reported by Schlagenhauf et al, revealed that gp63 with the β-sheet secondary structures belongs to the metzincin class zinc proteinase. The gp63 455070 dimensions have 478 amino acid residues. The protein structure consisted of three domains; N-terminal, Central and C-terminal as shown in Figure 4.
Figure 4: Ribbon representation of the gp63 structure with N-terminal domain, Central and C terminal domain shown in red, green and blue color respectively. Disulfide bonds shown in yellow and active site zinc atom is represented as sphere.
The N-terminal domain of protein contains Zn and the active site have catalytic residues such as His264 and His268. In addition, in metal ligation His264 and His268 are involved with the atoms of Zn+2. The central domain encompasses His334 (third catalytic residue), a Met-turn (Asp342-Asp348), α-helix C (H12), and 62 amino acid insertions (Phe272-Ser333) between Gly271 and His334. The extensive C-terminal domain of gp63 protein exist mainly the anti- p ll l β-strands and the random coil structure with only the slightly helical assistance. In the catalytic active site of gp63, zinc atom is correlated with the nitrogen atoms of His264, H 268, nd H 334 d nc of 2 18Ȧ, 2 18Ȧ nd 2 12Ȧ p c v ly (Schlagenhauf, et al. [11]). If the 3-dimensional structure of gp63 of Leishmania specie does not exist, there would be no correlations between the 3D structure and function of its defence as a vaccine. Razzazan, et al. [10], reported that in view of the development of molecular modeling bioinformatics, a model of gp63 structure with the help of homology modelling with the high precision can be predicted. Afterwards, analysis of the 3D structure of gp63, which can depict the exact information about its structure, function and its interactions (Razzazan, et al. [10]). Gp63 plays a central role in number of host cell molecular events that likely contribute to the infectivity of Leishmania. Furthermore, due of its abundance and ability to mediate resistance against infectious promastigotes, it has been suggested as a promising candidate for vaccination against leishmania infection.
Treatment for Leishmaniasis
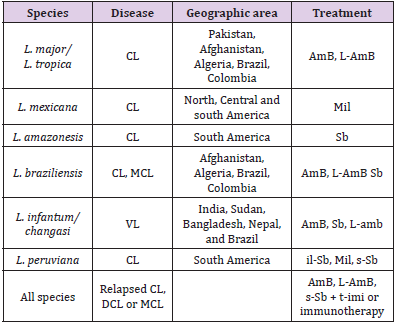
Treatment options used for leishmaniasis disease syndromes are intra-lesional stibogluconate, systemic stibogluconate, ketoconazole, fluconazole, Miltefosine, topical paromomycin, intramuscular paromomycin, liposomal amphotericin B, amphotericin deoxycholate, cryotherapy, heat therapy, Pentamidine, pentoxyfylline, allopurinol, topical imiquimod reported by McGwire and Satoskar. Table 1 Shows the Leishmanial diseases, parasites and treatment regimens by regions (McGwire, et al. [12]).
Plants as Remedy
Nature has provided many things for humankind over the years, including the tools for the first attempts at therapeutic intervention. Folk society use plant extracts for the treatment of various conditions. Nowadays, plant constituents stay a vital source for contesting illnesses, comprising contagious ailments. Variety of plants have been investigated for novel drugs or used as templates for the isolation of new therapeutic agents, food flavorings, agrochemicals and industrial chemicals (Lamchouri, et al. [13]). Almost all parts (leaves, stem, roots, seeds, flower and fruits) of the plant are helpful and used for the isolation of natural ingredient. The phytochemical is a natural bioactive compound found in plants, such as vegetables, fruits, medicinal plants, flowers, leaves and roots that work with nutrients and fibers to act as an resistance system against disease or more accurately, to protect against disease. Phytochemicals are categorize as primary like, common sugars, proteins, amino acids and chlorophyll while the second group include, alkaloids, carotenoids, terpenoids and phenolic compounds and many more such as flavonoids and tannins. The beneficial medicinal effects of plant materials typically result from the combinations of secondary products present in the plant. So the systematic screening of plant species with the purpose of discovering new bioactive compounds can help us to cure many fungal and bacterial diseases of economically important crops. The plant chemicals have been found to possess biocidal activity against several pests and pathogens. These are superior to synthetic pesticides in a number of ways like low mammalian toxicity, target specificity and biodegradability (Chugh, et al. [14]). Development in phytochemical studies of medicinal plants for pharmacological as well as nutritional purpose has ruling long time ago. Phytochemicals isolated from plants include essential oils, fixed oils, proteins, flavonoids, phenolic compounds and antioxidants serving as biocontrol agents. Extensive research has been conducted to identify the activity of medicinal plants against various diseases (Samiullah, et al. [15]) (Table 2).
According to world health organization (WHO) more than 80% of the world’s population relies on traditional medicine for primary health care needs. 250,000-500,000 plant species only a small percentage has been investigated phytochemicals and even few number submitted for biological and 2 pharmacological screening (Zain, et al. [16]). Access to hospitals and other medical abilities is inadequate and therefore, population depends mainly on local customary healers, when they get sick. These healers use various methods in their practice but use preparations of plants which is very successful and nominated practice since ages (Boulanour, et al. [17]). These preparation serves as real drugs, which have direct action on the body and can help to heal (cure) a certain disease (Sameulsson, et al. [18]). Almost every rural communities depending absolutely on medicinal plants for treating diseases and now most often urban population starts recurring to medicinal plants remedies, due to more expensive modern medicines (Boulanour, et al. [17]). Actually, more than 50% of drugs help for years as direct source or may as chemically altered natural products. China (consider as inventor of herbal medicine), India (the Middle East), especially Arab-Muslim World, Egypt, Greece and Rome those civilization used medicinal plants as a huge impact. Herbal-derived components with certain alteration (synthetic / chemical) serve as commercial medications used now-a-days world-wide against various treatments (Miguel, et al.). Due to climatic and phytogeographic conditions in Pakistan help to increase floral diversity containing many medicinal plant species. According to reports 6000 verity of floral species exist in Pakistan. Herbal remedies used in folk medicine provide an interesting and still largely unexplored source for the creation and development of potentially new drug for chemotherapy.
Conclusion
In the new world, leishmaniasis is a serious public health problem. Considering the drug development resistance worldwide, it is vital to achieve new, effective, safe and affordable drugs for parasite disease treatment. Natural source such as plants could be used for production of new antileishmanial agents against leishmania.
For more Articles on: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.