5HT1A Receptor Transcripts are Found in Retzius Cells; Interneurons Inhibit Neuritic Regeneration by Serotonin
Introduction
Following CNS injury, the organism loses its movement abilities to various degrees depending on the injury´s extension. This problem´s complexity has led to a dearth of information on the processes and elements that participate in CNS regeneration and recovery from injury. Clinically, this is reflected in the lack of totally effective treatments that could induce neuritic regeneration in injured neurons and hence, to the functional recovery of patients. Many factors play a role in neuritic regeneration such as neuronal identity, the extracellular matrix, microglia, and soluble factors among which, neurotransmitters are pivotal. The neurotransmitter serotonin has multiple functions [1], including a role in the neuritogenesis of hippocampal, cortical, spinal, and invertebrate neurons [2-4]. It has been reported to promote neuritogenesis [5,6], and to possess inhibitory properties [7,8]. Likewise, its receptors are determinants of the neurotransmitter´s functions. There are 7 types, and all have various sub-types [9]. As the neurotransmitter, its receptors have been associated with neuritogenesis [10-12], and various types of these receptors regulate the process [12-15]; there are also reports referring that R5HT1A inhibits neuritogenesis [16].
These observations indicate that the regenerative properties of various neuronal types may depend on the receptor they express, and in particular, they suggest that the inhibition of neuritogenesis by serotonin in RZ neurons is due to the presence of the 5HT1A receptor. The leech CNS has been used as a model to approach and attempt to answer various neurobiology questions and support this type of research, since its CNS transcriptome is present systemically [17], and in single, identified neurons [18]. The CNS is formed by 32 ganglia, the 21 intermediate ganglia with approximately 400 neurons, that regulate and control the organism´s functions. In every ganglion, Rz interneurons harbor serotonin and control many features of the animal´s behavior; AE motor neurons control the circular muscle annuli of the body, and the AL1 neurons possess great neuritic regeneration capacity. These 3 neuronal types respond differently in culture to serotonin by extensively growing or inhibiting their growth according to their identity [19]. In this study, we cloned and designed probes for the 1A and 2 serotonin receptors, to hybridize their genes in the CNS ganglia; we demonstrated that RZ neurons possess transcripts for the serotonin 1A receptor. These results suggest that Rz neurons decrease their neuritic growth in the presence of serotonin by activating the 5HT1A receptor.
The Phylum Annelida was used for this study: Haementeria officinalis is a Mexican leech from our Neuronal Regeneration Laboratory animal colony (Figure 1A). The procurement and maintenance of Haementeria officinalis samples have been previously described [19]. These organisms were originally collected in the lakes and dams of the central Mexican plateau by a researcher authorized by the local “Direction of Agricultural Development”. All procedures were approved by the Institutional Committee for the Care and Use of Laboratory Animals (INRCICUAL No. 18/18). The organisms weighed between 0.5 and 0.8g. The animals were anesthetized with 9% ethanol, for 20 minutes. They were fixated with pins on a wax dish, and the ganglion chain was exposed (Figure 1B), extracted, and fixated in a dish with silicone to conduct the hybridization procedures. The probes were designed based on the Hirudo medicinalis leech transcriptome data, to detect the neuronal transcripts [17].
Figure 1:
A) Image of a Mexican leech of the species Haementeria officinalis. Dorsal image.
B) Diagram of ventrally opened CNS. Its ganglion chain formed by 21 intermediate ganglia can be observed, as well as the ganglia fused in the head and tail, in the extreme ends of the ganglion chain. The arrows point to the ganglia from the 7th to the 11th. Scale bar=1cm.
For leech Haementeria officinalis R5HT1A cDNA probes were used. Fragment of R5HT1A (from Hirudo medicionalis transcriptome [17]), leech genes were obtained by RT-PCR. First-strand cDNA was synthesized with a First-strand cDNA Kit (Roche Applied Science) and 1μg of RNA of the ganglion chain from leech weighed between 0.5 and 0.8g. The following primers (5′to 3′) were used: R5HT1A 5′primer, 5 ́-TCGTCCAACTGCACTCTCTC-3′ and 3′primer 5′-AGGGGTTGAAGAGGCTGTTG-3′ (corresponding to region 166- 368). PCRs were performed in a total volume of 25 μl using Taq DNA polymerase (Invitrogen). The cycling conditions were 15 seconds at 94°C for denaturation, 30 seconds at 55°C for annealing, 1 minute at 72°C for elongation, and then 30 minutes at 72°C after the last cycle (35 cycles). The PCR products were cloned into pCR®-Blunt IITopo ® vector (Invitrogen 45-0245). The long of the fragments was confirmed by restriction assay.
Digoxigenin 11 UTP-labeled single-stranded RNA probes were prepared using a DIG RNA labeling kit (Roche Applied Science) according to the manufacturer’s instructions. Tissues from ganglion chain from leech were treated with 1μg/ml proteinase K (preincubated at 37°C) for 5 minutes at room temperature, then washed with PBT and incubated with hybridization buffer at 65°C for 15 minutes, after corresponding Digoxigenin-labeled probe was added and incubated overnight at 65°C. Next morning, samples were washed and incubated with AP-labeled anti-digoxigenin antibodies at 4°C overnight. Next day, sections were washed and revelated with BM Purple and observed under a Zeiss Discovery Microscope, and photographed with a Zeiss digital camera and processed with Zen blue software (Zeiss).
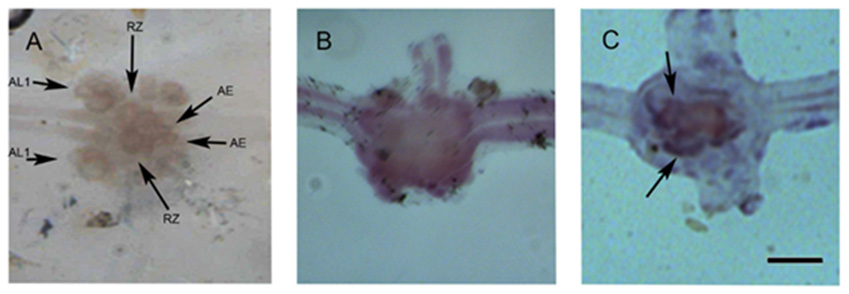
We made a probe that recognizing the 5HT1A receptor transcript for in situ hybridization. The probe that was designed and synthesized based on the transcriptome of the leech consortium [17] was functional, and binded to the Rz neuron. It was developed with anti-digoxigenin and detected with X-Purpura. The results were obtained from nine intermediate ganglia between the 7th and 11th ganglions from 3 different leeches. On figure 3, three ganglia are showed. The RZ, AL1 and AE neurons are in the ganglion without label (Figure 2A). On Figure 2B, the ganglion labeled with sense probe. The pair of RZ neurons that express the 5HT1A receptor had the label of antisense probe (Figure 2C. Arrows).
Figure 2:
A) Ganglion showing various identified neurons such as Retzius (Rz) interneurons, anterolateral 1 (AL1) neurons, and body annuli erector motor neurons (AE). The attached image shows a ganglion. In each ganglion, 2 sectioned nerves known as connective, are observed: the left one is anterior and to the side of the animal´s mouth while the right one is posterior, to the side of the tail. Laterally from each ganglion, 2 pairs of sectioned nerves are observed; they innervate the animal´s body. B) Hybridization with the sense probe.
C) Hybridization of the antisense probe of the 5HT1A receptor in an intermediate ganglion, developed with X-purpura. The ganglion in image A was hybridized with an “antisense” probe that recognizes the transcript of the 1A serotonin receptor. It is recognized by the arrows on the RZ neurons. Scale bar=100μm.
Conclusion
The probe that we obtained was functional and recognizes by hybridization some neuronal types, including Rz neurons; this suggests that these cells contain the transcript for the serotonin 1A receptor. This probe was designed on the basis of the leech transcriptome [17] and was inserted in the pCRR – Blunt II – topo R vector. The probe measures 202 bp that correspond to the 166-368 region of the 5htr1a gene of Hirudo medicinalis. Although the probe was designed based on the transcriptome of the Hirudo medicinalis leech, it also recognized neuronal transcripts of a different species: Haementeria officinalis, revealing the similitude in the sequences of the 5HT1A receptor in both species. Retzius neurons contain the R5HT1A transcripts, suggesting that this receptor´s expression leads to neuritogenesis inhibition mediated by serotonin. Thus, this receptor may play a role in the regulation of neuritogenesis in these neurons, as previously reported in other preparations that describe an inhibitory effect of the 1A serotonin receptor on neuritogenesis [16].
The inhibition of neuritic regeneration may be mediated by the 5HT1A receptor´s induction of a current promoting potassium entry into the cell, leading in turn, to a state of hyperpolarization that induces the inhibition of neuronal electric activity [20,21]. Further, electric activity is a mechanism regulating neuritogenesis via intracellular calcium regulation [22], and neuritic regeneration is inversely proportional to the calcium concentration in the neurite growth cones [23]. In the future, specific blockage of this receptor could be able to modify the regeneration of Rz neurons. However, we must test for the presence of other serotonin receptors, also in the Rz neurons. In other neurons, the final response may be mediated by an equilibrium in the activation of the serotonin receptors [14,15]. It is very relevant to understand the fundamentals of neuritic inhibition in Retzius neurons to evaluate its performance in organisms in vivo and in controlled culture conditions.
For more Articles on: https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.