Liquid- Liquid Extraction of Zinc(II) From Acid Media with N-N-Heptylaniline as an Extractant: Analysis of Pharmaceutical and Commercial Sample
Abstract
The extraction of Zinc(II) from acid media by N-n-heptylaniline in
xylene has been studied for metal distribution measurements. Various
physicochemical parameters like acid concentration, reagent
concentration, equilibrium time, effect of diluents, aqueous to organic
phase ratio, stripping agents and loading capacity were investigated for
the quantitative extraction. (The extraction was carried out from 3 M
hydrochloric acid and 0.5 M N-n-heptylaniline concentration). Zinc(II)
was selectively extracted and separated from many metal ions and
synthetic mixtures. The nature of the extracted species was determined.
Zinc(II) was analyzed from pharmaceutical samples and nycil talk powder.
Introduction
The uses of Zinc(II) are in the production of die-casting alloys, in
galvanizing industry, pharmaceutical samples, in the manufacture of
brass products, rolled Zinc(II) products of various types, light metal
alloys, in desilvering lead and in wet-batteries. It is starting
material for the production of Zinc(II) oxide. Zinc(II) is an essential
trace element in plant and animal life. The Zinc(II) content in humans
is 2 - 4g [1]. Zinc(II) is also essential constituent of several enzymes
necessary for metabolism. It is necessary to develop the simple, rapid
and selective method of separation for Zinc(II) from different elements.
For extraction and separation of Zinc(II) solvent extraction is useful
method. Solvent extraction of zinc(II), indium(III), thallium(III) and
bismuth(III) with n-octylaniline from hydrochloric acid media and their
separation carried out [2].
The distribution equilibrium of Zinc(II) between synergistic mixture
of N-n-octylaniline and trioctylamine in xylene and thiocyanate media
has been investigated [3]. The solvent extraction of Zinc(II) from
thiocyanate and sulphuric acid media using N-n- hexylaniline in xylene
is described [4]. Solvent extraction of zinc from strong hydrochloric
acid solution with alamine 336 has been carried out [5]. Liquid-liquid
extraction of Zinc(II) by 3-methyl- quinoxaline-2-thione from nitrate
medium investigated [6]. Separation of iron and Zinc(II) from manganese
nodule leach liquor using TBP as extractant is studied [7]. Solvent
extraction utilized for the selective separation of Zinc(II) from other
elements in hydro metallurgical processing of resources [8]. Zinc(II)
chloride and hydrochloric acid extraction from solutions of high
Zinc(II) concentration by tri-n-butyl phosphate diluted in ShellSol2046
(an aliphatic solvent) has been studied with a combination of
experiments and mathematical Modeling [9]. From chloride solutions
solvent extraction of Zinc(II) carried out [10].
The mean centering of ratio kinetic profiles method was used for the
simultaneous determination of binary mixtures of Ni(II) and Zn(II) in
water samples, without prior separation steps [11]. Synergistic
extraction of Zinc(II) by mixtures of primary amine N1923 and cyanex 272
investigated [12]. The solvent extraction of zinc(II), cadmium(II) and
chromium(III) from phosphoric acid solutions by tri-n-butyl phosphate in
kerosene as diluent was investigated [13].The extraction and separation
of zinc(II), manganese, cobalt and nickel from nickel laterite bacteria
leach liquor were carried out using sodium salts of TOPS-99 and Cyanex
272 in kerosene [14]. The selective removal of Zinc(II) over iron (II)
by liquid-liquid extraction from spent hydrochloric acid pickling
effluents produced by the Zinc(II) hot-dip galvanizing industry was
studied at room temperature [15]. Extraction and separation of cobalt
and Zinc(II) was studied from a sulphate solution using NaD2EHPA,
NaPC88A and NaCyanex 271 of 0.04M concentration [16]. The liquid-liquid
extraction of Zinc(II) using D2EHPA as extractant has been investigated
in order to recover zinc(II) sulphate from an industrial effluent
produced by Votorantim Co. which contains several metallic species such
as cadmium, cobalt, iron, lead, calcium, magnesium, manganese and nickel
[17].
The extraction of Zinc(II) and cadmium(II) with mixtures of neutral
organophosphorus extractants and amine extractants has been investigated
[18]. Synergistic solvent extraction and transport of Zn(II) and Cu(II)
across polymer inclusion membranes with a mixture of TOPO and aliquat
336 carried out [19]. Extraction of Zn(II) from aqueous hydrochloric
acid solutions into alamine-336 m-xylene systems was studied [20].
3-hydroxybenzylaminobenzoic acid synthesized as a reagent for the
determination of Zinc(II) in various water samples [21]. Recovery of
both sulphuric acid and Zinc(II) bleed stream generated during the
electrowinning of Zinc(II) in the Zinc(II) refineries using
tris-(2-ethylhexyl) amine dissolved in kerosene has been investigated in
detail [22]. The extraction of Zn(II), Fe(II), Fe(III) and Cd(II) with
TMbutylphosphate (TBP) and commercial trioctyl phosphine oxide in
kerosene from chloride medium has been studied [23]. Solvent extraction
behavior of zinc(II), cadmium(II), mercury(II) and bismuth(III) with
n-octylaniline in different organic solvents from various aqueous acid
solutions has been investigated [24].
An accurate, inexpensive and less laborious liquid-liquid extractive
spectrophotometric procedure for the determination of Zinc(II) in
aqueous media has been developed [25]. Previously we have reported
number of solvent extraction methods for the quantitative extraction of
platinum group metals with amines [2639]. In present research paper a
systematic study of extraction of Zinc(II) with N-n-heptylaniline in
xylene has been carried out. The extracted complex species in the
organic phase Zinc(II) was back extracted with 0.5M ammonia solution
(2X10mL) and was determined complexometrically. Various parameters such
as acid-reagent concentration, equilibrium period, effect of various
diluents, enrichment study, loading capacity and diverse ions were also
studied. The study was also extended for analysis of synthetic mixtures
and pharmaceutical sample.
Experimental
Apparatus
Mettler toledo model-ML204 electronic balance with accuracy 0.0001g
was used for weighing. For pH measurement Elico digital pH meter model
LI-120 with combined glass electrode was used. All glass wares were
cleaned by acidified solution of potassium dichromate and finally rinsed
with water
Reagents
Standard Zinc(II) Sulphate Solution (1 mg/mL)
The stock solution of Zinc(II) was prepared by dissolving 5.49g of Zinc(II) sulphate (ZnSO4.7H2O)
in 250mL of distilled water containing 0.5mL of concentrated sulphuric
acid. The solution was standardized and diluted as required for working
purpose.
Ethylene Diaminetetraacetic Acid Solution (0.001m)
A solution of ethylene diaminetetraacetic acid (0.01 M) was prepared
by dissolving 3.722g EDTA in distilled water and diluting to 1000mL and
standardized complexometrically. Working solution of EDTA (0.001M) was
prepared by proper dilution.
Buffer Solution (pH 10)
A pH 10 buffer solution was prepared by adding 142mL concentrated
ammonia solution (sp.gr. 0.88-0.90) to 17.5g ammonium chloride and
dilute it to 250mL with distilled water
N-N-Heptylaniline Solution (0.5M)
N-n-heptylaniline was synthesized by the method of Z. G. Gardlund
[40] and its solution (%, v/v) was prepared by using xylene as the
diluent.
General Procedure
To an aliquot of solution containing 1mg of zinc(II), add sufficient
quantity of hydrochloric acid to make the concentration of 3M in a total
volume of 10mL. Transfer the solution into a 125mL separating funnel
and shake the solution for 1 minute with 10mL of 0.5 M
N-n-heptylaniline. The swirl the solution and allow to separate the two
phases. After phase separation, strip the Zinc(II) from organic phase
with two 20mL portions of 0.5 M ammonia solution. Collect and combine
the aqueous extract and estimate Zinc(II) complexometrically [41].
Results and Discussion
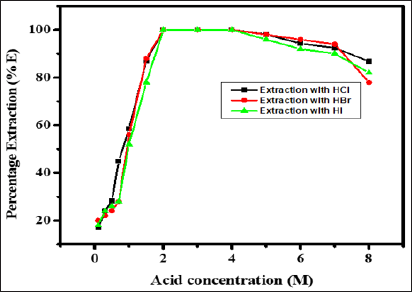
Influence of Acid Concentration on Extraction of Zinc(II)
The extraction of 1mg of Zinc(II) was studied from hydrochloric,
hydrobromic, hydroiodic, sulphuric, nitric and perchloric acid media in
the range of 0.1 to 8.0 M with 0.5 M N-n-heptylaniline in xylene keeping
the aqueous to organic volume ratio 1:1. The extraction of Zinc(II)
increases with increase in acid concentration and becomes quantitative
in 2.0 to 4.0M hydrochloric, hydrobromic and hydroiodic acid. While
Zinc(II) was not extracted with remaining acids. Upon further increase
in hydrochloric, hydrobromic and hydroiodic acid concentration the
extraction of Zinc(II) decreases (Figure 1), 3.0 M concentration of
hydrochloric acid was used throughout the work, as it has a wide range
of applications as compared with hydrobromic and hydroiodic acid.
Figure 1: Extraction behaviour of Zn(II) as a function of
hydrochloric, hydrobromic and hydroiodic acid concentration, Conditions:
Zn(II) = 1mg, N-n-heptylaniline = 0.5M in xylene, aq.: org. ratio =
1:1, shaking time = 1min, strippant = ammonia buffer (2 *10mL).

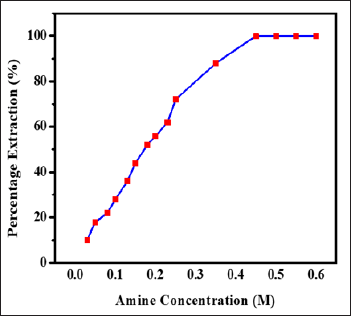
Influence of Extractant Concentration on Extraction of Zinc(Ii)
The effect of extractant concentration was scanned for the range of
0.03 M to 0.6M of N-n-heptylaniline on the 1mg Zinc(II) from 3M
hydrochloric acid. Increase in N-n-heptylaniline concentration was found
to be increase the extraction of zinc(II). The excess of reagent
concentration had no adverse effect on magnitude of extraction (Figure
2). It was found that, 10mL of 0.5 M N-n-heptylaniline was sufficient
for the quantitative extraction of 1.0mg of Zinc(II) from 3.0 M
hydrochloric acid. Therefore, in the recommended procedure 0.5 M
N-n-heptylaniline in xylene has to be used to ensure complete extraction
of zinc(II).
Figure 2: Influence of amine concentration on extraction of
Zinc(II), Conditions: Zn(II) = lmg, HCl = 3.0M, aq.: org. ratio = 1:1,
shaking time = 1min, strippant = ammonia buffer (2 *10mL).

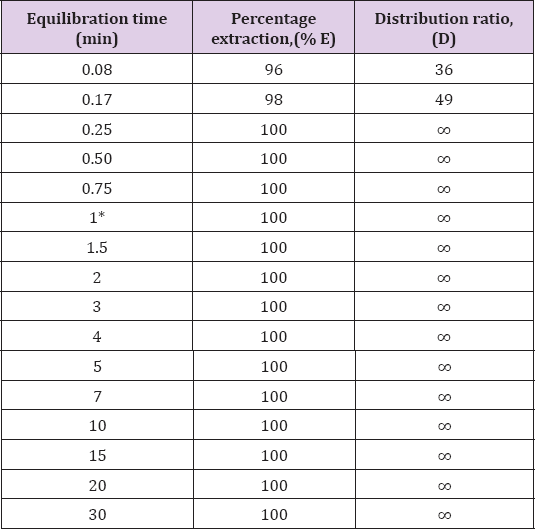
Influence of Contact Time on Extraction of Zinc(Ii)
The effect of phase contact time on the distribution ratio for the
extraction of Zinc(II) from 3 M hydrochloric acid using 0.5M
N-n-heptylaniline in xylene has been studied (Table 1). Variation of
shaking time from 10 s to 20min showed that a minimum of 30s shaking is
needed for the complete extraction of zinc(II). The prolonged shaking
had no adverse effect on the extraction, hence 1min shaking is
recommended in the general procedure to ensure the quantitative
extraction of zinc(II).
Note: *Recommended for general extraction procedure
Conditions: Zn(II) = 1mg, N-n-heptylaniline = 0.5M in xylene, HCl =
3.0M, aq.: organic ratio = 1:1, strippant = ammonia buffer (2 x10mL).
Extraction with Various Diluents
According to Sole appropriate hydrocarbon mixture used as diluents,
having flash and boiling point above 600 C and density of 0.8g / cm-3
to aid phase separation. The extra ctability and selectivity for the
extraction of metal ions by organic extractant are greatly affected by
the nature of solvent. A number of different diluents were tested for
the extraction of zinc(II). To discern the effect of nature of various
aromatic and aliphatic diluents on the extraction of Zinc(II) the
organic diluents namely xylene, toluene, benzene, nitrobenzene, carbon
tetrachloride and chloroform (Table 2). Out of these diluents, Zinc(II)
was quantitatively extracted in the xylene, toluene, benzene and
nitrobenzene while the remaining solvents give incomplete extraction of
zinc(II). Among this xylene was preferred as a diluent for further
studies since it provide better phase separation, relatively lower
aqueous solubility, ready availability and relative low cost.
Note: *Recommended for general extraction procedure
Conditions: Zn(II) = 1mg, N-n-heptylaniline = 0.5M in xylene, HCl =
3.0M, Shaking time = 1min, strippant = ammonia buffer (2 x10mL).
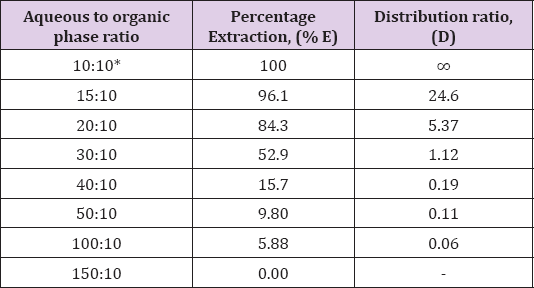
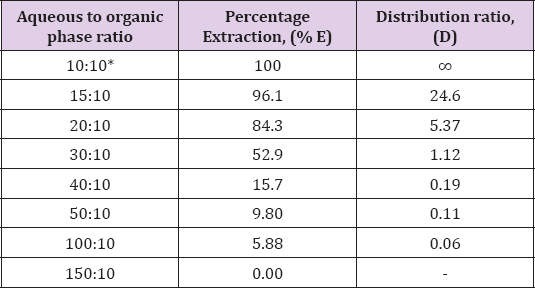
Effect of Aqueous to Organic Volume Ratio
To find out whether large aqueous: organic volume ratio could be
tolerated for the successfully extraction of Zinc(II) by N-n-
heptylaniline under optimum condition. Zinc(II) was extracted from
aqueous (10 to 150mL) of 3.0M HCl with 10mL of 0.5M N-n- heptylaniline
in xylene. Zinc(II) was stripped and determined as described in the
general procedure. It was found that extraction of Zinc(II) was
quantitative when aqueous to organic volume ratio was only 1:1 and it
decreased beyond it this may be attribute to the less stability of ion
pair formed under conditions (Table 3).
Note: *Recommended for general extraction procedure
Conditions: Zn(II) = 1mg, N-n-heptylaniline = 0.5M in xylene, HCl =
3.0M, Shaking time = 1min, strippant = ammonia buffer (2 x10mL).
Influence of Loading Capacity of N-N-Heptylaniline
The loading capacity of N-n-heptylaniline in xylene was studied by
equilibrating Zinc(II) in 3M hydrochloric acid at fixed aqueous to
organic ratio (1:1) for 1min. The two phases were separated, and the
same organic phase was again used for the extraction of fresh zinc(II).
The extraction of Zinc(II) by same N-n-heptylaniline was repeated till
no further extraction of Zinc(II) was observed in the organic phase. In
the saturated organic phase of N-n-heptylaniline the amount of Zinc(II)
was found to be 5mg.
Nature of the Extracted Species
Stoichiometry of the extracted species was determined by plotting Log
D[Zn(II)] versus Log C[HCl] at 0.05 and 0.25M N-n-heptylaniline
concentration having slopes 0.73 and 0.64 respectively (Figure 3).
Similarly, the graph of Log D[Zn(II)] versus Log C[N-n-heptylaniline] at
2.0M and 6.0M HCl concentration having the slopes of 2.03 and 1.69
respectively (Figure 4). The stoichiometry of the extracted species is
calculated to be 1:1:2 (metal: acid: extractant).
Figure 3: Log-Log plot of distribution ratio versus N-n-
heptylaniline concentration at 2.0M and 6.0M hydrochloric acid,
Conditions: Zn(II) = 1mg, N-n-heptylaniline = 0.5M in xylene, aq.: org
ratio : 1:1, shaking time = 1min, strippant = ammonia buffer (2 x10mL).
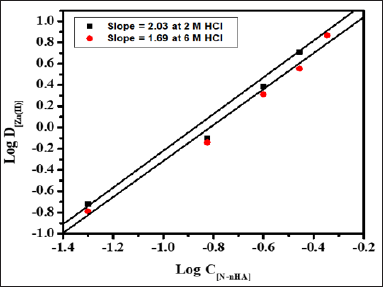

Figure 4: Log-Log plot of distribution ratio Log D[Zn(II)]
versus Log C[HCl] at 0.05 and 0.25M N-n-heptylaniline, Conditions:
Zn(II) = 1mg, aq.: org. ratio = 1:1, shaking time = 1min, strippant =
ammonia buffer (2 x10mL).
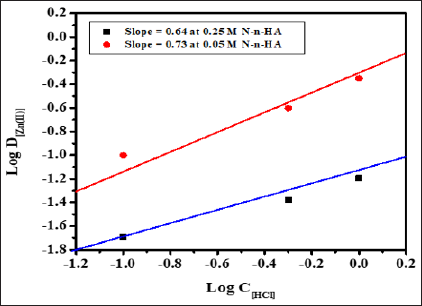

Hence the probable extracted species is: [(RR' NH2+)2 ZnCl42-] and the extraction mechanism appears to be,
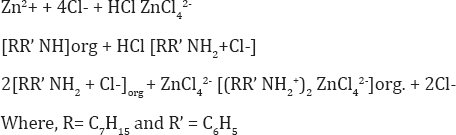
Applications
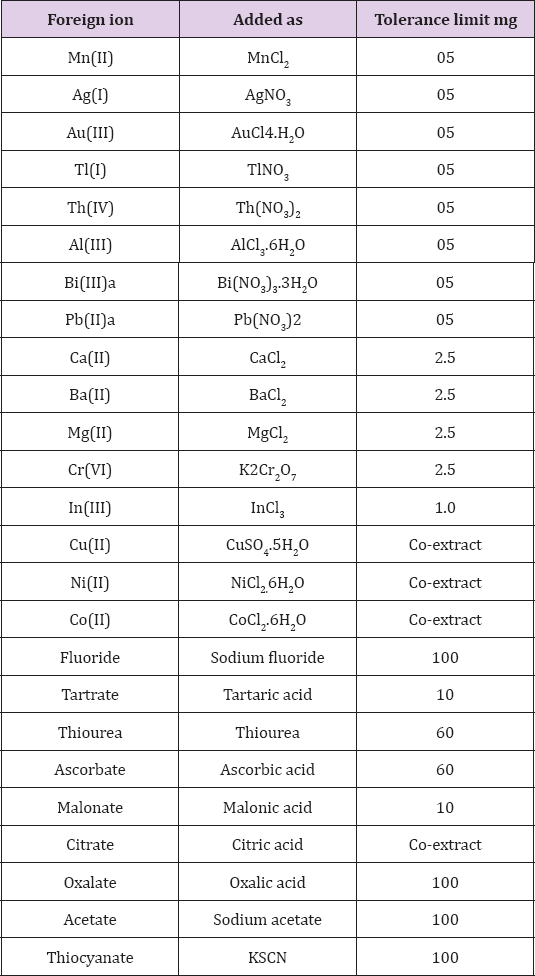
Influence of Foreign Ions
A number of ions were tested for the interference in the extraction
of Zinc(II) and complexometric determination. Varying amounts of foreign
ions were added to 1mg of Zinc(II) (in 10mL of solution) and
recommended extraction procedure was followed. There was no interference
from (as shown by less than 1.0 % deviation of Zinc(II) recovery) from
5mg each of manganese(II), silver(I), gold(III) and thallium(I),
aluminium(III); 2.5mg each of calcium(II), beryllium(II), magnesium(II),
chromium(VI); 100mg each of fluoride, acetate, thiocyanate; 60mg each
of thiourea, ascorbate, 40mg each of thiosulphate, 10mg each of
phosphate and malonate. Copper(II), nickel(II), cobalt(II), mercury(II),
bismuth(III) and lead co-extract. Of which bismuth and lead were masked
with thiourea. The results are tabulated in Table 4.
Note: a = Masked with thiourea.
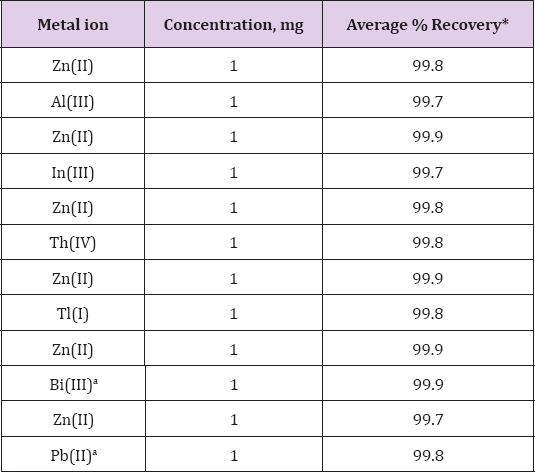
Separation of Zinc(II) From Binary Mixtures
In order to establish the validity of the proposed extraction
procedure, the method has been applied for the separation of Zinc(II)
from binary mixture. The binary separation of each metal ion was
achieved by selective extraction of Zinc(II) leaving behind other metals
in raffinate. The method was applied for the separation of Al(III),
In(III), Th(IV), Tl(I), Bi(III) and lead(II) Table 5. The extracted
Zinc(II) from loaded N-n-heptylaniline was stripped by washing the
organic phase using 0.5M ammonia.
Note:a = Masked with thiourea.
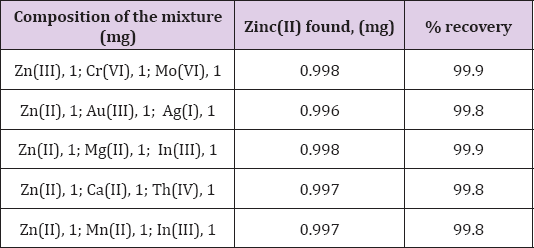
Separation of Zinc(II) from Synthetic Mixtures
Synthetic mixtures containing chromium(VI), gold(III), silver(I) in
addition to Zinc(II) are analyzed by the proposed method. Zinc(II) from
organic phase is back stripped with 0.5M ammonia and is determined
complexometrically. The results given in Table 6 show that the
separation of Zinc(II) is possible from synthetic multicomponent
mixtures.
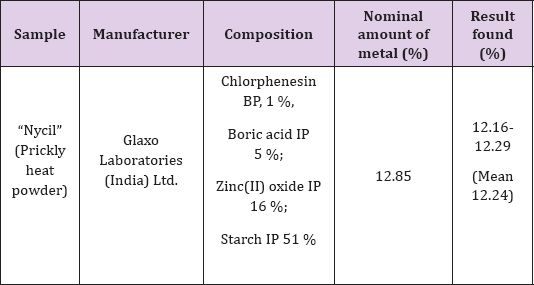
Analysis of 'Nycil' Talcum Powder
To 0.5g of sample placed in a flask add 2mL of conc. nitric acid and 10mL 3% KMnO4.
The mixture is heated on water-bath for 30min at 70-800C. Excess
permanganate was removed by adding solid oxalic acid. The solution was
diluted to 100mL and extract Zinc(II) as described in extraction
procedure. The results of analysis are reported in Table 7.
Note: *Average of six determinations.
Analysis of Zinc(II) from Pharmaceutical Samples
A tablet was dissolved in concentrated perchloric acid and solution
was evaporated to near dryness. The residue was taken up in the minimum
amount of perchloric acid and solution was evaporated to dryness again.
The residue was then leached with water and diluted to 100mL with water.
An aliquot was taken for the extraction and estimation of Zinc(II) by
the recommended procedure. The mean of six results is reported in Table
8.
Construct of Streptomyces Scabies Knock-Out Mutant of TXT Biosynthetic Gene txtB Via Intergeneric Conjugation - https://biomedres01.blogspot.com/2020/01/construct-of-streptomyces-scabies-knock.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.