Serious Adverse Events: What to Expect in Clinical Trials with Participation of Multiple Sclerosis Patients?
Abstract
Multiple sclerosis is an inflammatory disease attacking the central
nervous system, with a slightly reduced life-expectancy, affecting 2.1
million people worldwide, with female prevalence. A retrospective
analysis summarizes safety data from 11 completed clinical trials with
an aim to give an overview about Serious Adverse Event occurrence in
study subjects, also detailing the events according to System Organ
Class.
Abbreviations: SAE: Serious Adverse Event;
MedDRA: Medical Dictionary for Regulatory Activities; SOC: System Organ
Class; IMP: Investigational Medicinal Product; AEs: Adverse Events Introduction
Multiple Sclerosis
Multiple sclerosis is an inflammatory disease attacking myelinated axons in the central nervous system, destroying the myelin and the axon in variable degrees. It causes significant physical disability within 20-25 years in more than 30% of patients. The cause of multiple sclerosis is still unknown; it can be triggered or perpetuated by an as-yet-unidentified environmental factor in a genetically predisposed person. MS is considered an autoimmune disease; the immune system attacks the myelin sheets in the brain or spinal cord. Multiple sclerosis is most commonly diagnosed in people in their 20s and 30s. It is a life-time condition; average life-expectancy is slightly reduced. Worldwide, approximately 2.1 million people are affected, with prevalence in women [1].Clinical Trials
According to the definition in ICH GCP, in clinical trials only those adverse events (AEs) are assessed as serious (SAEs) that occurred after any dose of the Investigational Medicinal Product (IMP) and resulted in death, were life-threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability/incapacity or caused a congenital anomaly/birth defect. [2] Some of SAEs, which are assessed by investigators as related to the IMP, and also unexpected from regulatory standpoint - never registered before with the IMP - were accounted separately as they require expedited reporting to Regulatory Authorities, Ethics Committees, and Investigators participating in the studies with this IMP [2]. Pregnancies, which are actually not SAEs but require expedited reporting by the investigators and close follow-up until delivery and perinatal period of the baby were also included into account. A great deal of publications discusses the safety findings of clinical trials, but they do not specify serious events in detail. [3-6] This retrospective analysis provides an overview on the number and type of serious AEs in clinical trials with general multiple sclerosis patient population and is independent on the study drug or treatment arm.Retrospective SAE Analysis
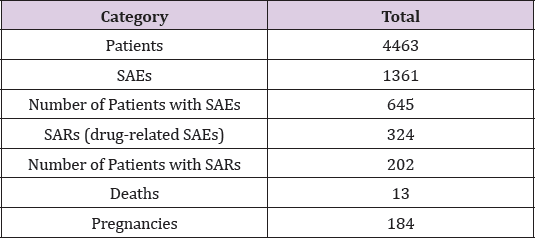
Retrospective analysis of SAEs was performed from data of 11 completed Phase II and III clinical trials completed with multiple sclerosis patients within the period of 2008-2018. A total of 4463 subjects in the age of 18 and 55, from Europe, Australia, North America and South Africa were enrolled in the studies (Table 1).During the analyzed trials 1361 SAEs were registered in 14.5% of treated subjects. One fourth of these SAEs were assessed as related to the investigational drug and unexpected (SARs), affecting almost 5% of the patients. A high amount of pregnancies was reported, 250 cases in 4.1% of all study subjects, in female subjects and female partners of male subjects. The most common seriousness criterion observed was hospitalization or prolongation of existing hospitalization. The most common disease groups according to System Organ Class (SOC) analysis using the Medical Dictionary for Regulatory Activities (MedDRA) were Infections and infestations and Nervous systems disorders. 10% of the reported SAEs were due to different sorts of infections: Pneumonia, Herpes zoster, Sepsis, Urinary tract infection, Cellulitis, Gastroenteritis and Appendicitis.
Note:
14.5% of all patients
23.8% of all SAEs
4.5% of all patients
0.3% of all patients
4.1% of all patients
The high occurrence of any kind of infection in MS patients is likely due to the fact that the drugs used for treatment have the common side effect of reducing white blood cell count. 8% of all SAEs (excluding cases of MS relapse) were connected to various illnesses of the nervous system. The explanation to their high occurrence can be either because of the inefficacy of the study drug or randomization into the placebo treatment arm. 13 subjects were died while in the trial, representing 0.35% of all treated patients.
The main reasons for death were due to Infections and infestations in 25% of the death cases. The rest of the cases belonged to the following disease groups: Blood and lymphatic system disorders; Neoplasms benign, malignant and unspecified; Nervous system disorders; Psychiatric disorders and Respiratory, thoracic and mediastinal disorders.
Conclusion
Fermentation of Pretreated Herbaceous Cellulosic
Wastes to Ethanol by Anaerobic Cellulolytic and
Saccharolytic Thermophilic Clostridia : https://biomedres01.blogspot.com/2020/01/journals-on-medical-informatics-bjstr_16.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.