Clinical Features and Treatment Strategy of Vasculitis-Associated Diffuse Alveolar Hemorrhage
Abstract
Objectives: To summarize the clinical features and diagnostic approach of patients with vasculitis-associated diffuse alveolar hemorrhage
(DAH). To investigate the optimal therapeutic strategy and highlight the effective corticosteroid dose and timing.
Methods: A retrospective chart review of the patients who were
admitted to the intensive care unit (ICU) due to vasculitis-associated
DAH was
performed. Patient characteristics, clinical manifestations, the
diagnosis of underlying etiology, treatment, and outcome were collected.
Results: During January 2015 to December 2017, seven
vasculitis-associated DAH patients were reviewed. The mean age ± SD was
53.4 ± 18.2
years. Five patients (71%) were female. DAH was the initial presentation
in all seven patients (100%). All patients required immediate
mechanical
ventilation. As first line therapy, methylprednisolone (MTP) pulse
therapy combined with cyclophosphamide pulse therapy were administered
to all
patients. Six patients survived. Four of them received MTP and
cyclophosphamide pulse therapy shortly after admission (mean 1.6 days,
range: <1-3days) showed good response to therapy and were extubated
successfully within ten days after ICU admission.
Conclusion: Vasculitis-associated DAH is a fatal disorder. Once the diagnosis of DAH is confirmed, intensive administration of MTP and
cyclophosphamide pulse therapy initiated within 3 days of admission provide good survival and pulmonary outcome.
Keywords: Alveolar hemorrhage; Corticosteroid; Lung Diseases; Therapeutic; Vasculitis
Abbreviations: DAH: Diffuse Alveolar
Hemorrhage; ICU: Intensive Care Unit; MTP: Methylprednisolone; RBCs: Red
Blood Cells; ANCA: Antineutrophil
Cytoplasmic Antibody; AAV: Associated Vasculitis; BAL: Broncho-Alveolar
Lavage; CXR: Chest X-Ray; SD: Standard Deviation; PTU: Propylthiouracil;
CRP: C-Reactive Protein; ESR: Erythrocyte Sedimentation Rate; IVIG:
Intravenous Immunoglobulin
Introduction
Diffuse alveolar hemorrhage (DAH) is an acute, lifethreatening
syndrome with clinical manifestations characterized
by hemoptysis, dyspnea, reduced hemoglobin, and diffuse
radiographic pulmonary infiltrations. The histopathology of DAH
involves the accumulation of intra-alveolar red blood cells (RBCs)
originating from the alveolar capillaries [1]. A broad spectrum
of disorders, including immune-mediated diseases, infections,
malignancies, and drugs, are all the possible underlying etiologies
of DAH. The most common clinical causes of DAH include small
vessel vasculitis, known as antineutrophil cytoplasmic antibody
(ANCA)-associated vasculitis (AAV), followed by Goodpasture
syndrome and other collagen vascular diseases [1,2].
Vasculitis-associated DAH typically presents with acute/
fulminant course and generally demonstrates high morbidity
and substantial mortality [3,4]. Therefore, prompt diagnosis and
aggressive treatment are required to improve survival. Recognition
of vasculitis-associated DAH depends on the awareness of clinicians;
once the diagnosis is established, the underlying etiology must be
investigated to initiate proper management. Delayed diagnosis
and insufficient treatment for the early stages of vasculitis-related
DAH may lead to irreversible pulmonary and extra-pulmonary
organ damage, particularly affecting the kidneys [5]. A combination
therapy of corticosteroid, cyclophosphamide, and plasma exchange
was recommended in vasculitis patients who present with
severe DAH [6,7]. However, the detailed therapeutic strategy (ex.
corticosteroid dosing and timing) and associated prognosis have
not been reported. The timing and sufficient dose of corticosteroid
for appropriate management of vasculitis-associated DAH remain a
challenge for clinicians in daily practice.
The aim of this present study was to describe the clinical
manifestations and prognosis of seven patients with vasculitisassociated
fulminant DAH, highlighting the diagnostic approach
and optimal therapeutic strategy.
Materials and Methods
We retrospectively reviewed the chart records of patients with
vasculitis-associated DAH who were admitted to the intensive care
unit (ICU) of Changhua Christian Hospital from January 2015 to
December 2017. The diagnosis of DAH was made on the basis of
at least three of the following: acute onset pulmonary symptoms
(dyspnea, hemoptysis), new infiltrates on chest radiographs,
reduced hemoglobin level, or bloody return on broncho-alveolar
lavage (BAL) with hemosiderin-laden macrophages in the absence of
macroscopic airway lesions [2,8,9]. Data collection included patient
characteristics, clinical manifestations (features of DAH, extrapulmonary
organ involvement, and laboratory assessment), the
diagnosis of underlying etiology, treatment, and outcome (patient
survival and pulmonary outcome). The Institutional Review Board
of Changhua Christian Hospital reviewed and approved this study.
Statistical Analysis
Basic demographic characteristics, clinical manifestations, laboratory
test results, chest X-ray (CXR), diagnosis, treatment, and
outcomes were reviewed and analyzed. Continuous variables were
expressed as mean ± standard deviation (SD) if normally distributed,
or as median (range) if skewed.
Results
Characteristics of Patients
Seven DAH patients were admitted into the ICU from January
2015 to December 2017. Three patients exhibited primary AAV,
two exhibited propylthiouracil (PTU)-induced AAV, one exhibited
IgA-nephropathy, and the remaining one exhibited Goodpasture
syndrome. The mean age ± SD was 53.4 ± 18.2 years. Five
patients (71%) were female. DAH was the initial presentation for
hospitalization in all seven patients (100%). The details are shown
in
Clinical Manifestations
Dyspnea was the most common symptom in all seven patients
(100%). Other pulmonary symptoms included hemoptysis
(in four, 57%) and cough (in one, 14%). All patients required
immediate mechanical ventilation, and overt bloody sputum from
the endotracheal tube was observed in all patients (100%). Renal
involvement with hematuria (in seven, 100%), proteinuria (in
six, 86%), and casturia (in one only, 14%) was present in these
patients. The mean ± SD serum creatinine level was 4.16 ± 4.37 mg/
dL (range: 0.42-11.54 mg/dL). Five patients (71%) suffered from
acute renal injury and four (57%) required hemodialysis. Renal
biopsy was performed in four patients.
Laboratory Data
Three patients experienced a reduction of hemoglobin within
48 hours after onset of DAH, while the remaining four patients
exhibited a low level of hemoglobin (5.9-8.6 g/dL) on admission;
however, these four patients lacked previous hemoglobin data
for comparison. BAL was performed in three patients (43%);
macroscopically hemorrhagic BAL fluid and microscopically
hemosiderin-laden macrophages were found in all three patients.
Bacterial, Mycobacterium tuberculosis, and fungal cultures, as well
as virus analyses, were all negative at the onset of DAH. All patients
were checked for C-reactive protein (CRP), procalcitonin, and
erythrocyte sedimentation rate (ESR) on admission. The mean ± SD
values were as follows: CRP, 12.65 ± 8.96 mg/dL (range 3.24-32.57
mg/dL); procalcitonin, 7.16 ± 11.01 ng/mL (range 0.08-33.15 ng/
mL); ESR, 79.71 ± 46.67 mm/h (range 12-140 mm/h).
Chest Radiography
CXRs showed bilateral alveolar opacities in all seven patients.
A unique pattern involving extensive, asymmetric, dense alveolar
opacities in both upper and lower parts of the lungs was present in
six patients (86%); this helped to distinguish alveolar hemorrhage
from pulmonary edema, which typically presented as butterfly-like
alveolar infiltration at CXR. We have included four patients with
different etiologies of DAH as examples; their CXRs on admission
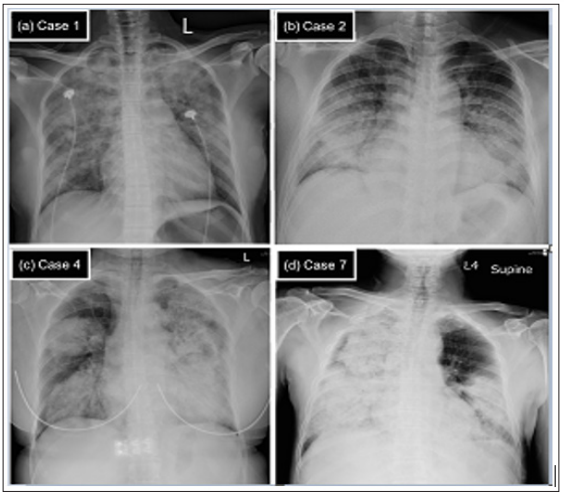
are shown in Figure 1.
Figure 1: The chest X-ray (CXR) on admission for four diffuse alveolar hemorrhage (DAH) patients with different underlying
causes.
Note: (a) Case 1. IgA-nephropathy. CXR showed extensive and asymmetric alveolar opacities in both upper and lower parts
of the lungs. (b) Case 2. Goodpasture syndrome. CXR showed bilateral symmetric peri-hilar alveolar opacities that mimic
pulmonary edema. (c) Case 4. Propylthiouracil-induced antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis
(AAV) and (d) Case 7. Primary AAV. For both (c) and (d), CXR revealed extensive, asymmetric, and dense alveolar opacities
and consolidation that involved whole portions of the lungs.
Diagnosis
The diagnosis of underlying etiologies for DAH in seven patients
was based on the clinical manifestations, laboratory test, and/or
histopathology of each patient. Five patients had a diagnosis of
AAV; all were diagnosed on the basis of clinical presentation of DAH,
positive serum ANCA result, and exclusion of other DAH etiologies.
Two of these five patients were diagnosed with PTU-induced AAV, on
the basis of drug exposure history; the other three were diagnosed
with primary AAV due to the lack of associated drug exposure or
other risk factors. Notably, in the remaining two patients (one with
IgA-nephropathy and the other with Goodpasture syndrome), blood
exams including anti-nuclear antibody, ANCA, and anti-glomerular
basement membrane antibody were negative; they were both
diagnosed by evident histopathology results on renal biopsy.
Treatment and Outcome
All patients required mechanical ventilation on admission
for respiratory failure; four patients required hemodialysis for
acute oliguria renal injury. Methylprednisolone (MTP) pulse
therapy (750 mg per day for 3 consecutive days) combined with
cyclophosphamide pulse (0.5-0.75 mg/m2, adjusted according
to renal function) therapy were administered to all patients as
first-line therapy. Plasma exchange was performed in six patients
(86%). Intravenous immunoglobulin (IVIG) (0.4 g/kg per day for
5 consecutive days) was prescribed as second-line therapy in two
patients (29%) whose illness was refractory to initial therapy. One
patient with remitting-relapsing DAH received rituximab therapy
(500 mg every week for 4 weeks). All patients received broad
empiric antibiotics and anti-fungal therapy, although there was
initially no positive culture result suggestive of major infection.
Throughout the course of hospitalization, no severe or overt
infection occurred in any of the patients. One patient (case 4) died in
the hospital; thus, the mortality rate was 14%. Initially, this patient
was treated conservatively by giving bolus corticosteroid (MTP 80
mg every eight hours) only. MTP pulse therapy was delayed until the 8th day after ICU admission; however, the patient died on the
next day after the first dose of MTP, due to uncontrolled DAH.
Six patients survived. Four of them (cases 1, 2, 3, and 5) showed
good response to therapy and were extubated successfully within
ten days after ICU admission; all four patients received MTP and
cyclophosphamide pulse therapy shortly after admission. For those
four patients, the duration from admission date to the first dose
of MTP pulse was 1-3 days (mean 1.6 days, range: <1-3 days). The
remaining two surviving patients reported prolonged ventilator
dependency, although they were free from active DAH. One patient
(case 7) began MTP pulse therapy 18 days after admission, followed
by intensive cyclophosphamide pulse and serial sessions of plasma
exchange. However, he experienced four recurrent episodes of
DAH during 2 months of hospitalization. Complete remission
of DAH was eventually reached after rituximab infusion. Due to
prolonged endotracheal intubation, he received tracheostomy
and remained ventilator-dependent. The other patient (case 6)
began a combination of MTP pulse, cyclophosphamide pulse, and
plasma exchange therapy on the day of admission, but her illness
was refractory to those therapies; second-line therapy of IVIG was
delayed until the 7th day after admission. Although DAH resolved
10 days after admission, she remained unconsciousness, likely
due to prolonged hypoxia encephalopathy. Detailed treatment and
prognosis information for the seven patients is summarized in
Table 2.
Discussion
This report was written to explore early and aggressive
treatment strategy and association with survival and pulmonary
outcome in patients with vasculitis-associated DAH. We found that
corticosteroid pulse plus cyclophosphamide pulse (0.5-0.75 mg/
m2
) in a timely manner (initiating administration within 3 days
of admission) provided better survival or pulmonary outcome for
patients with fulminant DAH. The corticosteroid dose should be
as sufficient as MTP 750mg daily for at least 3 consecutive days.
In contrast, delayed treatment after admission resulted in worse
pulmonary outcomes, including mortality.
Although early and aggressive treatment for DAH is important
for survival and prognosis, early recognition of DAH is challenging for
clinicians. The classical triad of DAH includes hemoptysis, anemia,
and asymmetric diffuse alveolar opacities at chest radiography.
DAH should be suspected when at least two signs of the triad are
present [10]. In our study, although all patients exhibited overt
bloody sputum from the endotracheal tube, apparent hemoptysis
before intubation was only present in 57% of patients. This is
consistent with previous reports [1,8,11]. Since hemoptysis may be
absent clinically, a decrease in hemoglobin without a clear cause
is suggestive of DAH [1,10]. Further, the majority of our patients
showed bilateral extensive alveolar opacities at CXRs, which
were helpful for consideration of DAH. Occasionally, opacities
located in peri-hilar or lower parts of both lung fields make it
difficult to distinguish DAH from pulmonary edema or infection.
In such situations, BAL plays an important role for the purposes of
Documentation of alveolar hemorrhage, finding hemosiderin-laden
macrophages from cytology analysis, and exclusion of infection
[12].
Notably, all of our cases showed a varying degree of renal
involvement; hematuria was the most common manifestation,
followed by proteinuria. Two cases made the definite underlying
etiology of DAH according to the characteristic histopathology of
renal biopsy (cases 1 and 2, IgA-nephropathy and Goodpasture
syndrome); both cases revealed negative findings in serum
immunologic tests. This suggests that biopsy of the damaged organ
helps to confirm the underlying etiology of DAH; the kidney may be
an optimal organ if active sediment, such as hematuria, is present
in urinalysis.
A combination of high-dose corticosteroid and cyclophosphamide
or rituximab has been established as the cornerstone of induction
treatment in small vessel vasculitis [6,13]. However, the
specific timing of therapy and optimal corticosteroid dose has not
been established. In our report, four patients who initiated MTP and
cyclophosphamide pulse administration within 3 days of admission
exhibited rapid remission from DAH within 1 week; they were
extubated successfully within 2 weeks. In contrast, two patients
(cases 4 and case 7) underwent delayed therapy due to missed
diagnosis of vasculitis and a concern regarding possible infection.
Actually, under the broad treatment of empiric antibiotics during
early stages of illness, no patient experienced severe infection. We
therefore report several good outcomes: first, in patients who were
confirmed to have fulminant DAH without overt previous systemic
diseases, vasculitis-mediated DAH remains the major cause. Second,
the “right treatment dose” at the “right time” provides good
prognosis for both survival and pulmonary outcome. The “right
dose” means not only a relatively higher dose of corticosteroid, but
the dose should be as high as the MTP pulse therapy (e.g., 750 mg
per day for at least 3 consecutive days), combined with cyclophosphamide
pulse (0.5-0.75 mg/m2
). The “right time” means that the
administration of MTP and cyclophosphamide should be started as
early as possible; we suggest that therapy within 3 days of admission
would be optimal timing.
Although there has not been sufficient evidence to support the
efficacy of plasmapheresis in patients with DAH, early plasmapheresis
to remove pathogenic antibodies has been recognized as an
effective and fast-acting adjuvant therapy in some studies [14-16].
However, there is no standard protocol of plasmapheresis for vasculitis-related
DAH. In our report, six (86%) patients received plasmapheresis,
concomitant with the administration of corticosteroid.
All of them showed severe organ damage at initial presentation,
either acute renal injury or fulminant DAH with severe hypoxemia.
We propose that prompt initiation of plasmapheresis is a good adjuvant
therapy, in addition to MTP pulse therapy, for patients with
fulminant vasculitis-related DAH.
There are limitations in our study. First, the heterogeneity of
the underlying vasculitis of our cases may indicate a diversity of
disease pathogenesis; this is compounded by variations in therapy
and prognosis. Second, due to the small case number in our report,
we cannot investigate other predictors or risk factors for prognosis.
Third, the long-term outcome could not be explored in our study. A
larger study with more patients and longer observation is required.
Fourth, in Taiwan, the use of rituximab is restricted by the high
cost and the policy of National Health Insurance and considered as
second-line therapy for patients who were failure to cyclophosphamide
treatment. Therefore, the first choice for those patients with
vasculitis-related DAH was limited by the combination of MTP pulse
with cyclophosphamide, rather than with rituximab in our report.
In conclusion, vasculitis-associated DAH is a fatal disorder. The
most common presenting symptoms are acute dyspnea, overt bloody
sputum from the endotracheal tube with reduced hemoglobin, and
extensive alveolar opacities at CXR. Renal involvement is frequent
and renal biopsy is helpful for evaluating the underlying etiology.
Although infection should play an important role, it should not be
viewed as an obstacle. Once the diagnosis of DAH is confirmed,
intensive administration of MTP (e.g., 750 mg daily for at least 3
consecutive days) and cyclophosphamide pulse therapy (e.g., 0.5-
0.75 mg/m2
, initiated within 3 days of admission) provide good
survival and pulmonary outcome.
A Mini Review on Some Latest Break Throughs
on Molecular Intervention for Human Diseases its Possible Effect
on the Brain Pathology - https://biomedres01.blogspot.com/2020/03/a-mini-review-on-some-latest-break.html
More BJSTR Articles : https://biomedres01.blogspot.com

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.