Clinical and Neurocognitive Outcome Evaluation In locked-in Syndrome
Introduction
Locked-in syndrome (LIS) is a rare condition in which patients are aware and awake but cannot move or communicate verbally due to complete paralysis of nearly all voluntary muscles in the body except for the eye movements. It is the result of a brainstem lesion in which the ventral part of the pons is damaged [1]. Ischemic strokes are the most common cause [2-6]. They most commonly occur following a basilar artery thrombosis with secondary occlusion of the perforating arteries. The other causes of LIS may be hemorrhages, brain trauma (pontine contusion or axonal damage), tumors. This syndrome is defined by five criteria (American Congress of Rehabilitation Medicine, 1995)
a) Sustained eyes opening and preserved vertical eye movement
b) Preserved higher cortical functions
c) Aphonia or severe hypophonia
d) Quadriplegia or quadriparesis
e) Primary modality of communication through vertical eye movements or blinking.
Corticospinal tracts damage (paralysis of all four limbs) induces selective supranuclear motor de-efferentation. Damage to the nuclei of the facial nerve and lower cranial nerves explains the presence of facial diplegia, anarthria, dysphagia, tongue paralysis, as well as aphonia and respiratory failure. The mesencephalon and the oculomotor nerve are intact. The age of onset of LIS varies between 17 and 52 years old. The actual prevalence rate of LIS is not specifically documented in the literature, probably represents less than 1% of all strokes, and the incidence rate is probably underestimated. During the acute phase, infections, most commonly pneumonia, are the most common cause of death (40% of the cases). The initial stroke is the primary cause of death in 25% of the cases. More than 85% of individuals are still alive after ten years [7-12]. Bauer described three categories of LIS [12,13].
a) Complete or total LIS: Quadriplegia and anarthria. No eye movement.
b) Classic LIS: Preserved vertical eye movements and blinking.
c) Incomplete LIS: Recovery of some voluntary movements in addition to eye movements.
The diagnosis of LIS may sometimes be difficult, above all during the acute phase, especially in cases of LIS caused by a traumatic brain injury with an initial coma. Some patients, at first, present complete LIS, in which no voluntary eye movement is possible. This clinical condition may thus lead to incorrect diagnoses of prolonged coma, vegetative state, minimally conscious state or akinetic mutism, although cognitive functions are generally preserved [14-19].
c) Incomplete LIS: Recovery of some voluntary movements in addition to eye movements.
The diagnosis of LIS may sometimes be difficult, above all during the acute phase, especially in cases of LIS caused by a traumatic brain injury with an initial coma. Some patients, at first, present complete LIS, in which no voluntary eye movement is possible. This clinical condition may thus lead to incorrect diagnoses of prolonged coma, vegetative state, minimally conscious state or akinetic mutism, although cognitive functions are generally preserved [14-19].
We studied a LIS male patient (age: 62 years old; education: 5 years) who suffered a pontine ischemic stroke. The patient presented spontaneous breathing, bilateral spastic quadriparesis, bilateral Babinski’s sign and aphonia. He had sustained eyes opening and movements (consistent vertical tracking and fixation) with response to visual threat. The brain Magnetic Resonance Imaging (MRI) examination showed ischemic lesions at pons level, and periventricular white matter hyperintensities. The delay since the initial event was 20 months. The patient underwent a clinical and neuropsychological evaluation compounded by several tests, assessing different cognitive functions and the quality of life (Table 1). The written informed consent was obtained from the patient’s legal guardian. The clinical and neuropsychological tools were adapted to get dichotomic responses. His YES-NO responses were based on a non-verbal communication channel, on closing eyes for YES and rising eyes for NO. He was assessed twice (at baseline – T0, and after two months – T1), in order to evaluate test-retest reliability, homogeneity and internal coherence. The clinical and neuropsychological assessment included:
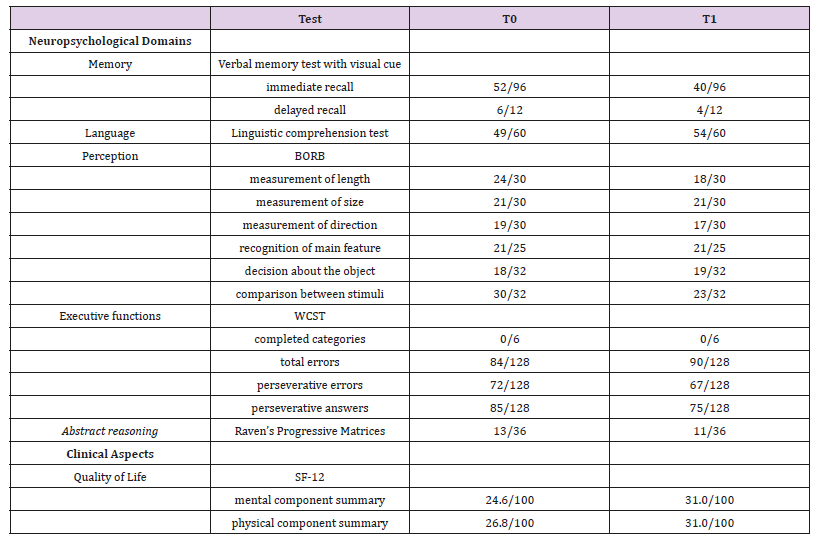 Note: BORB-Birmingham Object Recognition Battery; WCST-Wisconsin Card Sorting test; SF12-Short Form Health Survey
Note: BORB-Birmingham Object Recognition Battery; WCST-Wisconsin Card Sorting test; SF12-Short Form Health Surveya) Verbal memory test with visual cue (adapted from Test di Memoria e Apprendimento – TEMA) [10]. The patient was required to memorize a list of words read by the examiner and to remember them before immediately and then after a visuospatial task. In both steps, the immediate and delayed recalls occurred showing the visual cue, in which the patient had to indicate the target picture corresponding to word among distracters.
b) Linguistic comprehension test (adapted from Aachener Aphasie Test, AAT) [2]. This test evaluates the oral comprehension of words and sentences. The examiner read a word, or a sentence and the patient had to recognize and to indicate the numbered target stimulus picture corresponding to a word or a sentence among distracters.
c) Birmingham Object Recognition Battery (BORB) [23]. This test measures the perception and recognition of stimuli. Some subtests of BORB were administered, such as measurement of length, measurement of size, measurement of direction, recognition of main feature, decision to the object, and comparison between stimuli. All subtests involved decision making tasks among stimuli. We excluded some subtests, implying use of language or praxic skills.
d) Wisconsin Card Sorting test (WCST). This test assesses strategies planning ability, problem solving, judgement ability, cognitive flexibility. The patient was showed four numbered cards, the so called “guide cards” representing different drawings, a red triangular, two green stars, three yellow crosses and four blue circles. Then, the patient received other cards representing the same drawings arranging in different way. The patient was asked to associate each card to the “guide cards” according to a logical criterion (ex: color, shape, number) previously established by the examiner. The criterion is changed after ten right consecutive answers and the patient had to identify every time the right criterion.
e) Raven’s Progressive Matrices. This test is used to evaluate the abstract reasoning. The patient was showed the Raven’s progressive matrices. They consist of some drawings without a box and the patient was asked to choose which from the showed numbered pieces was appropriate to complete the drawing.
f) Short Form Health Survey (SF-12). This tool is a generic instrument to measure quality of life. It is the reduced version of SF-36. It consists of 12 questions focusing on eight domains: physical functioning, physical role, bodily pain, general health, vitality, social functioning, emotional role and mental health. Higher scores representing better health status or functioning (Table 1).
All clinical and neuropsychological total raw scores, at baseline and after two months, were compared to evaluate the following validity features: test-retest reliability assessed through the Intraclass Correlation Coefficient (ICC), homogeneity through the Pearson’s correlation coefficient and internal coherence through the Cronbach Alpha. The internal coherence is a measure of the internal consistency of a scale and it concerns the extent to which the items of a test or instrument are measuring the same thing. The homogeneity refers to the linear correlation of each item with the total score of a test. The individual items of an instrument measuring a single construct should give highly correlated results reflecting the homogeneity of the items. The test-retest reliability is a statistical measure refers to the accuracy of the repeated measuring of the clinical and neuropsychological performance in different moments of time. The ICC was used as the index of agreement. We found ICC values above 0.80, considered indicative of good agreement.
In LIS patients, most of the previous investigations have emphasized the preservation of cognitive functions have reported normal intelligence and performance in the spatial orientation, right-left discrimination, language, and calculation tests in a patient suffering from LIS for 12 years, who was assessed by using a writing device through a yes/no code at the eye level. Two further studies showed preservation of verbal comprehension (Boston Diagnostic Aphasia Examination) and intelligence (Wechsler scale). Conversely, the longitudinal investigation of another case revealed impairment of processing speed, attention and concentration, and functioning on performance-based tests. Disorders of perceptual aptitudes and executive functioning were also demonstrated. These problems were partially recovered over time. Furthermore, the study of long-term LIS survivors identified attention and memory disorders as relatively frequent complaints. Our results seem to be consistent with the literature data, indicating the absence of extensive cognitive deficits in LIS patients, but the presence of a specific impairment in one or more tests.
In conclusion, this study, even if limited to a single patient, shows that cognitive functions may be investigated clinically in a LIS patient who is able to use a yes/no code, and who presents with moderate and selective cognitive disorders. Therefore, from these preliminary data, even if limited to one patient, we can hypothesize that this specific clinical and neuropsychological battery has a significant degree of reliability and so it could be validated for LIS patients, even if more data are requested. As future perspective, we propose to enhance the sample investigating the sensibility and specificity of this clinical and neuropsychological battery for LIS patients.
More BJSTR Articles : https://biomedres01.blogspot.com

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.