Introduction
Hyperlipidemia, increased serum cholesterol and triglycerides, as a metabolic disease results from impaired lipid metabolism by excessive intake of cholesterol or genetic deficiency. High blood cholesterol levels are caused by kidney diseases, diabetes, medication side effects, hypothyroidism, metabolic syndrome, polycystic ovary syndrome (PCOS) and Cushing’s syndrome [1,2]. Hyperlipidemia not only causes the progress of atherosclerosis primarily as a result of increased serum cholesterol levels, but also leads to systemic inflammation that can be effective on disease development [3-5]. A variety of studies reported the effects of dietary fats on diseases such as age-related memory loss [6], infertility and endometriosis [7,8] multiple sclerosis, breast cancer, prostate cancer, colon cancer and non-Hodgkin’s lymphoma [9- 14]. The immune system is a complex array of organs, tissues and specialized cells that protects us from outside invaders and tumor initiation in body. Studies to determine which metabolic disorder or combination of disorders in obese people increases their cancer risk have been inconclusive [15]. High level serum lipids lead to altered rates of cholesterol in the cell membrane and cytoplasm of macrophages. Therefore, hyperlipidemia might inhibit innate immune responses, cytokines release and inflammatory response and impair proper immune response to bacterial challenge [16] and increase risk of cancer [17,18]. On the other hand, in vitro studies show an increase in the number of IL-4 producing Th2 cells [19] and a decrease in Th1 response and cell immunity in ApoE-/- mice [20-22].
By the measurement of cholesterol levels in patients with superficial esophageal cancer, Akihiro Sako et al. suggests that high levels of serum lipid might provide suitable conditions for the progress of lymph node metastasis in the early stage of esophageal cancer [23]. In contrast, some previous studies demonstrate low serum levels of cholesterol and triglycerides (TG) could lead to cancer morbidity [24,25]. Although all the studies about the effects of hyperlipidemia on immune response were dispersed, its relation to cancer is still controversial. We hypothesized that hyperlipidemia could reduce Th1 related cytokines and increased risk of cancer in human. For considering this purpose we decided to investigate the effect of high level of cholesterol and LDL on cell mediated immunity and expression of Th1 related cytokines in human.
Study Population
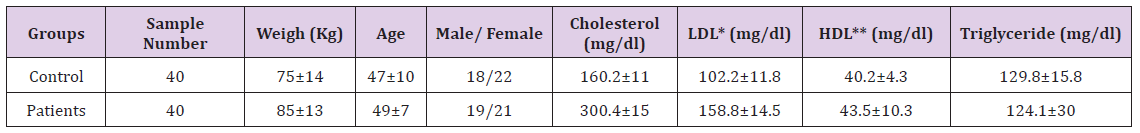
Forty untreated hypercholesterolemia patients in the clinic department of Hazrat Rasoul hospital and forty healthy individuals joined this study. The diagnosis of hypercholesterolemia was based upon an appropriate clinical history and measurement of LDL rate per weight. They all gave written informed consent, and the local ethics committee approved the study protocol.
 Note: (*Low Density Lipoprotein, ** High Density Lipoproteins)
Note: (*Low Density Lipoprotein, ** High Density Lipoproteins)The patients who smoked or had other current inflammatory, infectious diseases or neurological disorder, cardiovascular, diabetes and allergic diseases were excluded from the study. The control group were healthy individuals and they had no other hyperlipidemia diseases (Table 1).
We collected 4 ml of peripheral blood samples from the cubital vein of two study groups of normal controls and patients in EDTA-containing tubes. Then, peripheral blood mononuclear cells (PBMCs) were separated from total blood samples based on gradient density centrifugation technique by Ficoll hypaque (Pharmacia, Uppsula, Sweden).
The total cellular RNA was extracted from PBMC by High Pure RNA Isolation Kit (Roche, Germany) according to the manufacturer’s instructions. To normalize RNA concentration, we measured its absorbance by using the Nano Drop 2000 instrument (Wilmington, USA) at 260nm. The RNA (400ng/μl) from each sample was used to synthesize the first-strand cDNA by the cDNA synthesis kit (Fermentase, Germany). Similarly, cDNA synthesis was carried out based on the manufacture’s protocols. Ultimately, the cDNA samples were kept at -70oC until we used them for PCR.
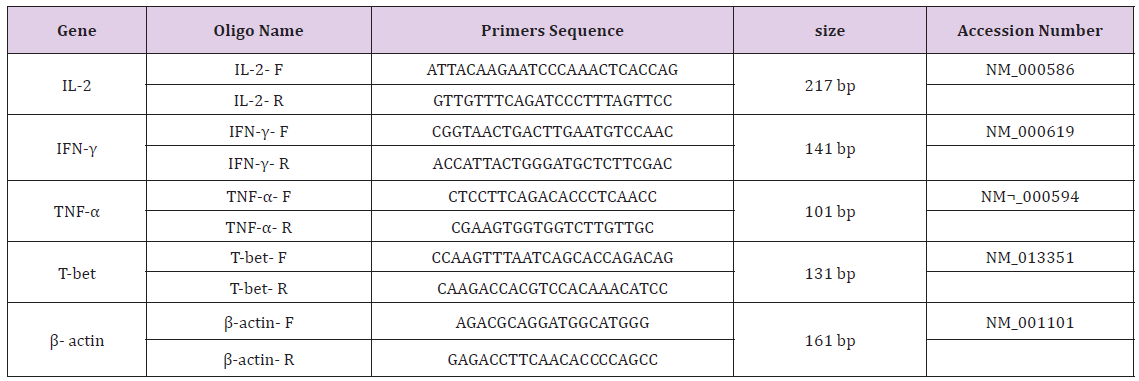
Primers were designed using oligo7 software (WWW.oligo. net). To exclude amplification of genomic DNA and pseudo genes we confirmed the validity of the primers by blasting: http://www. ncbi.nlm.nih.gov/tools/primer-blast/ (Table 2). A common PCR technique was carried out for all samples in a final volume of 20μl with master mix PCR (Cinnagen, Tehran, Iran). Then the samples were loaded on a 1.5% gel. Real-time-PCR was performed with SYBR® Green fluorescent dye (Light Cycler Fast Start DNA Master Plus SYBR Green I, Roche, Germany) to scan cDNA amplification by binding only to double stranded DNA and its fluorescent intensity was identified by Rotor gene (Termocicler Rotor-Gene™6000 Corbett Research/ Australia). The melting curve analysis showed only one peak for each reaction and this was also confirmed by electrophoresis of PCR products that showed only one band of the expected size.
 Statistical Analysis
Statistical AnalysisData output from LinReg PCR software such as Cycle of threshold (Ct) and efficiency of each reaction were imported to Relative Expression Software Tool 2009 version (REST 2009).
This study was performed on 40 untreated hyperlipidemia patients and 40 healthy people. It was important which hyperlipidemia patients have high level of cholesterol and LDL only while HDL and triglycerides level have to be normal. We investigated the effect of high level of cholesterol and LDL on T cell differentiation and cell mediated immunity.
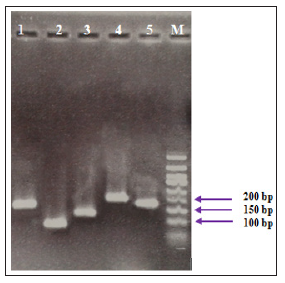
The electrophoresis of PCR products associated to the studied genes showed specific sharp bands for IFN-γ (141 bp), TNF-α (101 bp), T-bet (131 bp), IL-2 (217 bp) and β-actin (161 bp), without any primer dimers (Figure 1).
 Hypercholesterolemia Decrease mRNA Expression of Th1 Related Cytokines
Hypercholesterolemia Decrease mRNA Expression of Th1 Related CytokinesIn order to investigate the effect of Hypercholesterolemia on Th1 related cytokines, the mRNA expression levels of IL-2, IFN-γ and TNF-α cytokines were measured by real time PCR. As reference gene for loading we used the housekeeping gene β-actin. The results showed a significantly decrease in mRNA expression of IL-2, IFN-γ and TNF-α cytokines by 0.056, 0.310 and 0.285 respectively. The differences of cytokines and transcription factor of the patients group in compared with healthy individuals were statistically significant (P≤ 0.006) (Table 3) and (Figure 2).
T-bet is an important transcription factor to differentiation of Th0 to Th1 and regulated expression of Th1 related cytokines. In order to investigate the effect of Hypercholesterolemia on Th1 differentiation, the mRNA expression levels of T-bet transcription factor were measured by real time PCR and β-actin housekeeping gene was used as reference gene. The result showed that Hypercholesterolemia lead to down regulated mRNA expression of T-bet transcription factor by 0.439. The differences of the two groups were statistically significant (P≤ 0.006) (Table 3) and (Figure 2).
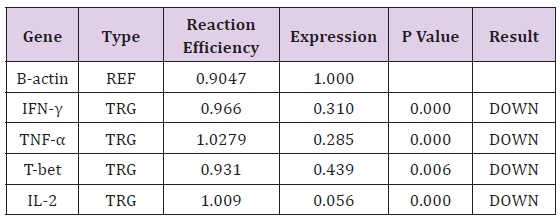 Note: (TRG-Target, REF-Reference).
Note: (TRG-Target, REF-Reference).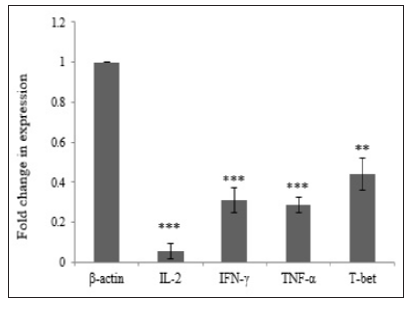 Discussion
DiscussionDyslipidemia, in particular high cholesterol and hyperlipidemia, is considered as an important metabolic disease throughout the world that is significantly associated with inflammation [26,27]. Hyperlipidemia begins partial inflammation by change of leukocyte activity and disrupting cytokine regulation, which worsens over time. Many studies have reported the effects of hyperlipidemia and dietary fats on immune responses, however, many of them have been carried out on animal models with focus on innate immunity. A deeper understanding of hyperlipidemia effects on the immune system will provide a more comprehensive investigation of the health risks of hyperlipidemia and risk of infectious diseases and cancer. In order to get an understanding of hyperlipidemia on the cell mediated immunity, we investigated the effect of high level of cholesterol and LDL on the Th1 cell differentiation. Many studies have reported weakened immune defense for ApoE-/- hypercholesterolemic mice against Candida albicans, Listeria monocytogenes, Klebsiella pneumonia and lymphocytic choriomeningitis virus (LCMV) infection [28-31].
More BJSTR Articles : https://biomedres01.blogspot.com

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.