Preliminary Blood Compatibility Comparison of Silk Fibroin Dissolved by different Solvents
Introduction
Haemorrhage still remains a leading cause of early death and infectious complications in trauma or combat wounds continue to challenge caregivers. Various materials (such as gelatine, fibrin glue, chitosan, collagen, and inorganic microporous materials) have been developed as haemostatic materials [1-5]. On market currently, gelatine and chitosan have attracted several clinically proven products for wound haemostasis. Collagen is also considered a promising biomaterial for wound haemostasis [1,5], but it do not have the ability to induce the rapid hemostasis required for surgical applications [6,7]. Chitosan, as a haemostatic material, is able to stop low pressure arterial bleeds, but is flimsy to maintain hemostasis after intravenous administration of fluids, leading to the risk of rebleeding [8]. After modification, the haemostatic efficiency of chitosan can be improved [9,10]. Collagen, gelatine and chitosan do not have physiological haemostatic functions.
Materials and Methods
Preparation of Silk Fibroin Solution
Raw silk was treated with boiling Na2CO3 solution to remove sericin, as described previously [16]. After drying, degummed silk fibre was dissolved in (1) a ternary solvent CaCl2·CH3CH2OH·H2O (mole ratio, 1:2:8) at 70 °C or 9.3 M of LiBr at 60 °C or molten Ca(NO3)2 at 85 °C and stirred until completely dissolved. After dialyzing against deionized water, concentrating and filtering the mixed solution to obtain silk fibroin aqueous solution (6%, w:w).
6% of silk fibroin aqueous solution were mixed with polyethylene glycol diglycidyl ether (Sigma, USA) at a weight ratio of 1.0: 0.8(w/w) and stirred to ensure entirely mixing. The mixture was cast into 6-well cell culture plate, then prefrozen at -80 °C for 8h and freeze-dried for 24h to prepare porous materials. After impregnation in deionized water at 4 °C for 72h to remove unreacted residues, porous materials were freeze-dried again. Regenerated silk fibroin materials were named to SF-CaCl2, SF-LiBr, SF-Ca(NO3)2, respectively. Commercial calcium alginate porous material was used as a control.
Specimens were equilibrated in saline (0.9%,w:v; NaCl) at 37 °C for 30min. Whole blood from a healthy rabbit (2.5kg, New Zealand; the Laboratory Animal Research Centre, Soochow University, China) was collected into sterile sodium citrate buffer [25], then the specimens were soaked in diluted rabbit blood (0.2ml in 10ml saline) to incubate at 37 °C for 60min. Distilled water and physiological saline were used as positive and negative controls, respectively. After centrifuging at 2500rpm for 5min, the absorbance of the supernatant was recorded at 545nm using a Synergy HT microplate reader (BIO-TEK, USA). The Hemolysis percentage was calculated according to the following equation, where A1, A2, and A3 are the absorbance of the negative control, sample, and positive control, respectively. All samples were analysed in triplicate.
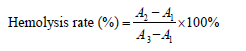
Coagulation Time Testing
Fresh blood from a rabbit (2.5kg, New Zealand) was collected and mixed with sterile sodium citrate. Specimens were placed in mixture solution and incubated at 37 oC for 30min. After removing samples, the Platelet-Poor Plasma (PPP) supernatant was collected by centrifuging at 3000rpm for 5min. Activated Partial Thrombin Time (APTT), Prothrombin Time (PT) and Thrombin Time (TT) were determined according to the instructions supplied with the relevant kits (Nanjing Jian cheng Bioengineering Institute, China). Briefly, APTT assay, 50μL PPP supernatant was mixed with 50μL APTT reagent and incubated at 37 oC for 3min, followed by the addition of 50μL preheated CaCl2 (0.03mol/L) and mixing; PT assay, 50μL PPP supernatant was incubated at 37 oC for 3min, then mixed with 100μL preheated PT reagent; TT assay, 100μL PPP supernatant was incubated at 37 °C for 3min and mixed with 100μL TT reagent.
Whole blood in sodium citrate buffer was centrifuged at 1500rpm for 15min to obtain Platelet-Rich Plasma (PRP) supernatant. Specimens were pre-equilibrated with saline for 24h and further incubated with PRP at 37 °C for 3min, then added 0.025M CaCl2 to accelerate recalcification at 37 °C. PRP supernatant without materials was used as control group. The time taken for fibrin clots to appear was recorded as the PRT. All samples were analysed in triplicate.
Data are presented as mean ± standard deviation of the mean. Comparisons of means were performed using one-way Analysis of Variance (ANOVA), followed by the independent sample t-test using SPSS 17.0 statistical software. A p-value lower than 0.05 was considered as statistically significant.
Results and Discussion
Hemolysis of Silk Fibroin
Figure 1: Hemolysis ratio (A) and hemolysis observation (B) of regenerated silk fibroin materials. a: distilled water; b: physiological saline; c: SF-CaCl2; d: SF-Ca(NO3)2; e: SFLiBr.
 Lithium salt (such as LiBr and LiSCN) and calcium salt (such as CaCl2 and Ca(NO3)2) are recognized solvents used to prepare regenerated silk fibroin materials [16,26,27]. In this work, we used LiBr, CaCl2 and Ca(NO3)2 to dissolve silk fibers. Figure 1 showed the haemolytic properties of regenerated silk fibroin porous materials. The Hemolysis ratios of regenerated silk fibroin materials prepared by different dissolution methods were all less than 2% (s 1A) and were therefore non-hemolytic [28]. All samples did not damage the red blood cells any more than did the negative control (physiological saline) (Figure 1B). In contrast, the positive control (distilled water) caused extensive damage to the red blood cells, which released large quantities of haemoglobin as a result.
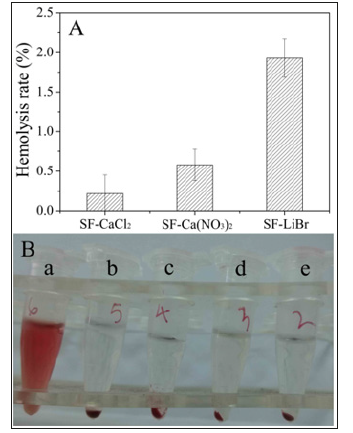
Lithium salt (such as LiBr and LiSCN) and calcium salt (such as CaCl2 and Ca(NO3)2) are recognized solvents used to prepare regenerated silk fibroin materials [16,26,27]. In this work, we used LiBr, CaCl2 and Ca(NO3)2 to dissolve silk fibers. Figure 1 showed the haemolytic properties of regenerated silk fibroin porous materials. The Hemolysis ratios of regenerated silk fibroin materials prepared by different dissolution methods were all less than 2% (s 1A) and were therefore non-hemolytic [28]. All samples did not damage the red blood cells any more than did the negative control (physiological saline) (Figure 1B). In contrast, the positive control (distilled water) caused extensive damage to the red blood cells, which released large quantities of haemoglobin as a result.Coagulation system is complex involving various factors that act on both intrinsic and extrinsic activation pathways. Herein, APTT, PT and TT were investigated to evaluate clotting capability of regenerated silk fibroin porous materials dissolved by different solvents (Figure 2). The PT value of SF-CaCl2 and SF-Ca(NO3)2 were shorter than that of SF-LiBr, similar to calcium alginate (Figure 2A). Thromboplastin is the key factor in blood coagulation, in the PT test, there was no obvious difference in the results between samples due to the addition of sufficient thromboplastin and appropriate Ca2+. The APTT reflected the clotting ability depending on Ca2+ activating. Although silk fibroin dissolved with calcium salt contained a small amount of chelated Ca2+ and a little CaCl2 reagent were added, it is far less than that of calcium alginate. Therefore, the APTT values of silk fibroin materials were much longer than that of calcium alginate (Figure 2B). In the TT test by adding prothrombin without Ca2+, the TT values for silk fibroin materials dissolved by calcium salt was significantly shorter than that by LiBr, while slightly longer than that of calcium alginate (Figure 2C). In this test, Ca2+ is also indispensable and played a significant role to activate platelets to release thrombin activating factor, thereby converting prothrombin into thrombin. This Ca2+ was only derived from plasma or materials, so the TT values of SF-CaCl2 and SF-Ca(NO3)2 were shorter than that of SF-LiBr with significant differences, although there was only a small amounts of chelated Ca2+ in calcium salt dissolved silk fibroin materials.
 Recalcification of Silk Fibroin
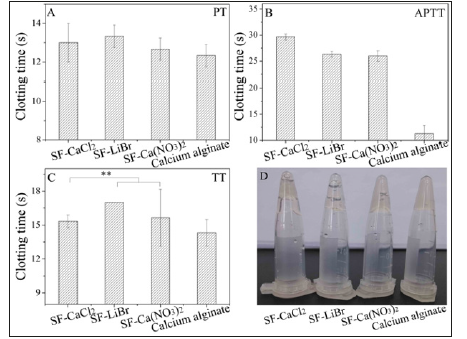
Recalcification of Silk FibroinFigure 3: Recalcification time of regenerated silk fibroin materials **p<0.01.
 Plasma recalcification time is a convenient measure of the endogenous anticoagulation properties of biomaterials. The recalcification time of regenerated silk fibroin materials dissolved with CaCl2 and Ca(NO3)2 was significantly shorter than that with LiBr (Figure 3) and that of control. The results also because of remained bivalent Ca2+, which chelated with -NH2 or -OH of silk fibroin, activated rich platelets to release thrombin activating factor and promote fibrin formation, resulting in shorter recalcification time of regenerated silk fibroin prepared by calcium salt dissolving.
Plasma recalcification time is a convenient measure of the endogenous anticoagulation properties of biomaterials. The recalcification time of regenerated silk fibroin materials dissolved with CaCl2 and Ca(NO3)2 was significantly shorter than that with LiBr (Figure 3) and that of control. The results also because of remained bivalent Ca2+, which chelated with -NH2 or -OH of silk fibroin, activated rich platelets to release thrombin activating factor and promote fibrin formation, resulting in shorter recalcification time of regenerated silk fibroin prepared by calcium salt dissolving.Conclusion
Silk fibroin molecules can chelate with Ca2+, a small amount of chelated Ca2+ remained in the silk fibroin materials by calcium salt dissolving and dialyzing. Results showed that the regenerated silk fibroin materials prepared with calcium salt dissolution had a prominent advantage for shortening clotting time compared with non-calcium salt reagent. The study provided an idea to develop silk fibroin haemostatic materials by using a more suitable preparation method, such as regulating the Ca2+ concentration.
Spider Bite Inducing Superficial Lymphangitis: A Case Report-https://biomedres01.blogspot.com/2020/10/spider-bite-inducing-superficial.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.