Prenatal Diagnosis of Dystrophin Gene Mutations using Multiplex Ligation Dependent Probe Amplification (MLPA) for Duchene Muscular Dystrophy
Introduction
Duchene muscular dystrophy (DMD) and Becker muscular dystrophy (BMD) are common X-chromosomal recessive disorders caused by mutations in the dystrophin gene. Male children are primarily affected by the disease, which is characterized by progressive muscular wasting and increased serum creatine kinase. The worldwide incidence of DMD is estimated to be about 1 in 3, 500 to 1 in 5,000 male births [1]. In Pakistan, the incidence has not been reported, however few isolated studies have reported the gene mutation analysis patterns in the both distal and proximal hot spots in the effected patients [2-3]. DMD and BMD genotypes are characterized by mutations in the dystrophin gene, which has 79 exons. Up to 70% of DMD are associated with deletions, and 5-10% with duplications in the gene. However, approximately 25% to 30% of boys with DMD, and 5% to 10% of boys with BMD do not have whole exon deletions or duplications but have other small insertions/deletions/point mutations.
Genetic conditions are broadly classified into those which are either dominant or recessive and may be associated with either autosomes or sex-chromosomes. If the disease is X-linked, then a male individual with an X and Y chromosome with a mutation on the X chromosome would necessarily be affected by the disease condition. However, a female individual with the mutation may not be affected to the same extent as they would have a normal X-chromosome to compensate for the mutation present. However, such a female individual who is heterozygous for the disease causing mutation may be termed as a ‘carrier’ for the disease [4]. As DMD is an X-linked disease, mothers with dystrophin gene mutations may be carriers who transfer risk for DMD to their off-spring [5-6]. Following that, female offspring may themselves be affected carriers while male offspring would definitely have DMD. Pre-natal testing performed during pregnancy can determine the genotype of the fetus. This allows the parents to make an informed decision to be prepared for a child with a likelihood for disease armed with knowledge that will help them to prepare and plan for the future or, to make a decision regarding the pregnancy outcome.
Different prenatal screening and diagnostic tests are possible. Chorion villus sampling (CVS) can be done at 10-12 weeks, and amniocentesis at about 14-16 weeks, while placental biopsy and fetal blood sampling can be done at about 18 weeks [7-8]. Currently the most widely used method for diagnosis of dystrophin gene mutation is based Multiplex Ligation-Dependent Probe Amplification (MLPA). In Multiple Probe Ligation and Amplification assay (MLPA), oligo-probes are hybridized to each dystrophin gene exon, followed by a ligation reaction. The ligated products are then amplified by polymerase chain reaction (PCR). Different length products are separated on an automated capillary sequencer where the amplified exon peaks are quantified. Deletions and duplications in the dystrophin gene are assessed by analysis software to determine the extent of mutations. Multiple Probe Ligation and Amplification assay (MLPA) is a powerful molecular tool for the detection of deletions or duplication in any of the 79 exons in the dystrophin gene. The test employed is a MLPA kits, SALSA P034-B1 and P035-B1 (MRC-Holland, Netherlands, CE-IVD marked) which can identify deletion/duplication mutations in 79 exons of dystrophin gene.
Case Report
We report a case in which a 24 years old lady gravida 2 and para 1+0 referred from Aga Khan Hospital Hyderabad for prenatal diagnosis in November 2017 to the Fetal Medicine Unit, Department of Obstetrics and Gynecology, The Aga Khan University, Karachi. The study subject presented at 12 weeks of gestation and had a previous history of a three-year-old male child affected by DMD. She was advised for pre-natal diagnosis of dystrophin gene mutation testing for which amniocentesis was performed at 16+1 weeks of gestation. In addition, a blood sample was taken from the study subject and also her son affected by DMD. Blood and amniocentesis specimens were submitted to the Section of Molecular Pathology, The Aga Khan University Clinical Laboratory for Dystrophin gene mutation testing by MLPA. First, a check for maternal cell contamination (MCC) was performed in the pre-natal sample in order to ensure that the diagnostic result would be robust and not likely to be influenced by carryover of any maternal DNA.
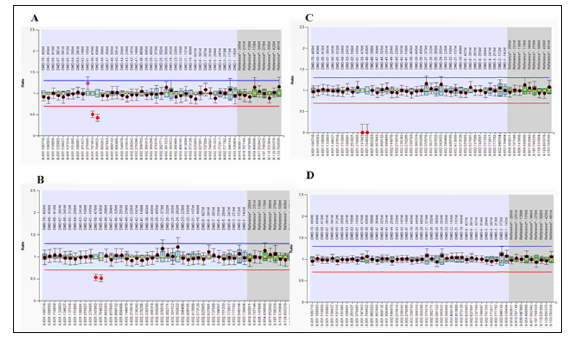
Figure 1 depicts the results obtained and reveals a heterozygous deletion in Exons 49-50 of dystrophin gene to be present in ‘A’ the pre-natal specimen and the ‘B’ the female patient. In addition, a deletion of dystrophin exons 49-50 was observed in the male child affected by DMDM ‘C’. Overall, the results indicated that the fetus, like her mother, would be a female carrier positive for dystrophin gene mutations. Also, that the affected male child had acquired the same mutation from his mother. Based on this result, the patient was referred back to her home town Hyderabad, Sindh, for further antenatal care. Unfortunately, the study subject did not have the recommended anomaly scan done at 20 weeks of gestation. Instead, a growth ultrasound was performed at 27 weeks of gestation which showed the evidence of hydrops fetalis. Due to this result, the family opted to discontinue the pregnancy and termination of pregnancy was performed at 28 weeks of gestation.
Figure 1: Detection of mutations in dystrophin gene Exons by MLPA. MLPA kits, SALSA P034-B1 and P035-B1 (MRC-Holland, Netherlands), were used to detect the deletion/duplication mutations in 79 exons of dystrophin gene in the Amnio, patient, DMD affected boy of the patient, and normal healthy control sample according to the instructions of MLPA kits. A, B: MLPA SALSA P034-B1 showed a dosage quotient (DQ) ratio of 0.5 in Exon 49-50 (heterozygous deletion) in both the pre-natal sample and the study subject. C: The DQ ratio 0 for Exon 49-50 (homozygous deletion) was observed in the sample for DMD affected child. D: Normal healthy control sample had a DQ ratio 1 for all Exon, no deletion or duplication was detected.
Discussion
DMD is a progressive genetic disorder. It an X-linked condition caused by a mutation in the dystrophin gene. This gene encodes the dystrophin protein and lies on the short arm of chromosome X (Xp21.2-p21.1). Mutations in this gene also give rise to a milder form of muscular dystrophy, with later onset, called Becker Muscular Dystrophy (BMD). Various mutations can give rise to DMD, but the most common are large deletions of sections of the gene, which are reported to account for two thirds of cases. About 61% of cases are caused by deletions, 13% by duplications, and 26% by point mutations or other small mutations. The deletions that give rise to DMD are often those that result in a frame shift, i.e. deletions that cause all the subsequent sections of the gene to be read incorrectly by the protein making machinery of the cell.
Conclusion
MLPA for dystrophin gene mutations revealed that amnio exhibited heterozygous deletion in Exon 49-50, same as observed that in the mother. The effected boy exhibited a homozygous deletion in Exon 49-50. Women have two X chromosomes, and therefore if they have only one X chromosome with a mutation in the dystrophin gene, the other copy of the gene may be able to compensate. Once a boy is identified as being affected by DMD or BMD, their mother should be assessed to see if she also carries the mutation [9-10]. Given that there is no available treatment, the greatest benefits of screening would be that a very small number of births of second affected boys would be averted. In the absence of a cure, prenatal diagnosis appears to be the best approach to reduce the burden of this disease on the individual family and ultimately on society.
Radiology. From Analogic to Digital-https://biomedres01.blogspot.com/2020/10/radiology-from-analogic-to-digital.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.