Enhancement of Anthocyanin, Chlorophyll and Total Phenolic Content of Buckwheat Seedling By The Application of Led Light
Introduction
Sunlight is one of the most important factors for plant growth and development. However, in the glasshouse, sometimes the intensity of sunlight is not enough for plants to grow well because of continuous overcast, rainy days, greenhouse structures and coverings [1]. Artificial LED light has been strategically used to improve the plant food quality especially polyphenols contents of the plant in a controlled environment [2]. It is aforementioned in many reports that blue and red light are vital wavelengths for plant growth and development [3]. The absorption percentage of blue and red light is about 90% than the other spectra [4]. Light is the basic factor for growth and development of plant [5]. Supplemental light stimulates plant growth, promote stem extension [6], and increase a dry matter of pepper [5] radish and lettuce [7] and increase the leaf number of Alternanthera brasiliana [8]. Researchers are found that light quality affects the phytochemical concentration of plants [9,10]. It has been reported that blue light increased anthocyanin levels in tomato [11], carotenoids in coffee [12] and ascorbic acid in lettuce plants. On the other hand, Alokam et al observed that lower red / far-red ratio (R / FR), or more FR relative to R light decreased anthocyanin concentrations in potato and alpine crops [13]. These results have shown the viability of optimizing light quality in increasing phytochemical concentration and growth of plants.
The aim of this work was to determine which wavelengths of the supplemental light emitting diodes (LEDs) work best for buckwheat plants to obtain high-quality vegetables with enriched phytochemical concentrations in glass house condition.
Materials and Methods
Buckwheat seeds were sown in a plastic tray and placed in a growth chamber maintaining temperature 25 0C and RH 50%. After germination (3 days after seeding) all trays were moved to the greenhouse and waited up to distinct two true leaves and then two trays were subjected to each supplemental light treatment (as described below) inside the greenhouse.
Supplemental Light Treatments
A LED light panel consists of 20 LED sticks (20 LED bulbs on a stick) with the main controller (33.5 cm wide, 27.5 cm long and 10 cm height, LPRS Series, Good Feeling Co. Ltd., Korea). LEDs light were placed horizontally 25 cm above the plant canopy. The experiments composed of five treatments with different supplementary LED wavelengths: red (R) 660 nm, blue (B) 470 nm, far-red (FR) 740 nm, a combination of blue and red light (B: R 1:1), and natural light treatment (without any supplementary lighting) as a control. Supplemental light duration was 16 h/day (5 a.m. to 9 p.m.).
Measurements
Data were collected (after 15 days of providing supplemental light) to measure the growth characteristics such as plant height, stem length, no. of node and leaf, leaf length, leaf width, and plant fresh weight. After growth measurements, the samples were freezedried and prepared for chemical analysis.
Determination of Ascorbic Acid
A modified protocol reported by Gahler et al. was used to determine the content of ascorbic acid [14]. Fresh samples (10g) were mixed with 40 mL of 5% Metaphosphoric acid and blended to extract ascorbic acid. The mixtures were shaken at 250 rpm for 5 minutes and then centrifuged at 3,000×g for 10 min. The supernatants were used to determine the concentration of ascorbic acid using the HPLC system equipped with a C18 column (Agilent Technologies 1200 Series), sample injects 3 μml, maintained at 300C. The extract was eluted with mobile phase (HCN 5%, DI water 95 % with 0.1% formic acid) at a flow rate of 0.5 mL/min, run time 10 minutes. The absorbance of the eluant was measured at 254 nm and concentrations were determined against ascorbic acid standards (Mallinckrodt Baker, Inc., Phillipsburg).
Total Phenolic Compounds
The method of Singleton and Rossi was used to determine total phenolic compounds [15]. Briefly, freeze-dried samples (50 mg) extracted were with 10 mL 80% methanol and shaken at 240 rpm for 16 h. After filtering 50 μL of the methanolic extract was then mixed with 350 μL of H2O and 200 μL of 1N Folin- Ciocalteu reagents (Sigma Chemical Co., St. Louis, Mo). The mixture was incubated for 1 h in 1.0 mL of 10% Na2CO3 at 250C. The absorbance of the incubated mixture was then measured at 735 nm a UV-5000 VIS NIR spectrophotometer (Varian Technologies, Australia) with a standard curve to estimate gallic acid (Sigma Chemical Co., St. Louis, Mo.) equivalent (GAE) concentrations.
Anthocyanin Analysis
Freeze-dried samples (30 mg) were extracted with 5 mL 2% HCl in methanol for 36 h. The liquid extract was separated by centrifugation at 1446×g for 15 min. For each sample, separate 400 μl aliquots of extract were diluted to 2.0 mL with two different buffer solutions: potassium chloride buffer (0.025, pH 1.0) and sodium acetate buffer (0.4 M, pH 4.5). After 15 min reaction, both solutions were filtered (0.2 μm pore size) and the absorbance was measured at 515 nm, where maximum absorption was confirmed in separate scans taken with UV-5000 VIS NIR spectrophotometer (Varian Technologies, Australia) and at 700 nm for haze correction. Total anthocyanin concentrations were expressed as cyaniding-3- glucoside equivalent values, following the protocol described by Giusti and Wrolstad [16].
Total Chlorophylls Content
Fresh leaf tissue (100 mg) was extracted in 5 mL N, N dimethylformamide overnight. The absorbance of extraction solution was measured at 647 nm and 664 nm a UV-5000 VIS NIR spectrophotometer (Varian Tech., Australia). Chlorophyll concentration was calculated using the equations described by Moran [17].
Statistical Analysis
All data were analyzed by one way ANOVA and Duncan’s multiple range test (DMRT) using MSTAT at 5% level of significance.
Results and Discussion
Effects of Supplemental Led Light Qualities on Growth Characteristics
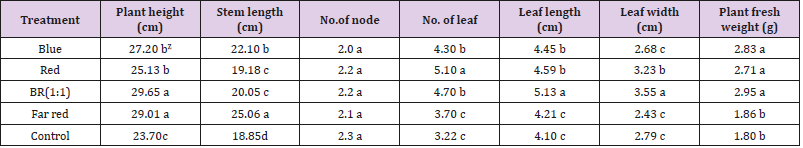
Growth characteristics of buckwheat plant were greatly influenced by different supplemental light treatments (Table 1). Plant height, Leaf length, leaf width, and plant fresh weight were increased by supplemental BR light. Far-red light increased the stem length and red light increased the leaf number compared to control. Blue and red lights are the major energy sources for plant growth and development [18]. Previously blue and red lights were proven as an effective lighting source to grow lettuce in a controlled environment [19]. Moreover, the positive effect of red light on the growth of perilla, tomato, chrysanthemum, and various herbs was studied [20]. It is widely understood that phytochrome photoreceptors enhanced the plant growth and development through activation of HY5`s gene which is stimulated by red light [21]. Martinez-Garcia et al. also illustrated that red light activates the phytochrome photoreceptor which enforces the relocalization of the nucleus and modulates gene expression [22].
Table 1: Effects of supplemental LED light qualities on growth characteristics of buckwheat plants.
Note: ZMean separation within columns by DMRT at 5% level.
Effects of Supplemental Led Light Qualities on Phytochemical Contents
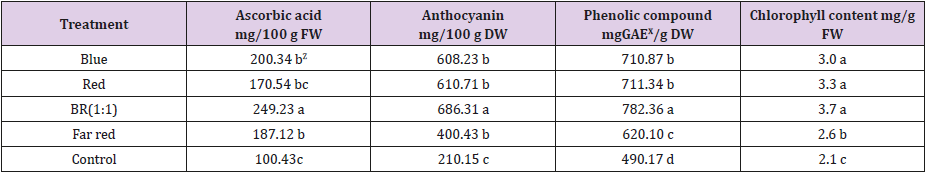
Phytochemicals concentrations in buckwheat were significantly affected by different light treatments (Table 2). Among the treatment BR light significantly increased the ascorbic acid, anthocyanin, phenolic compound of buckwheat plant. Along with BR treatment, B and R treatment also showed good performance compared to control. Total chlorophyll content of buckwheat plants was profoundly increased by BR light treatment. Plant pigments have specific light absorption spectra. For instance; Chlorophyll has high light absorption at 400-500nm [23]. Blue light is abundantly absorbed by photosynthetic pigments and an important catalyst to increase the Chl contents in many plants; including lettuce and cucumber [24]. Results from the current study show that buckwheat plants have higher Chl content grown under BR light which is consistent with the findings of Son et al.[19]. Ma et al. illustrated that key gene activity of the enzyme in Chl pigment is stimulated by blue light resulting in higher pigments accumulation [25]. The combination of BR light was an important lighting source for the accumulation of polyphenol compounds such as; anthocyanin, ascorbic acid and total phenolic compound in lettuce [26].
Table 2: Effects of supplemental LED light qualities on phytochemical contents of buckwheat plants.
Note: ZMean separation within columns by DMRT at 5% level, XGAE= Gallic Acid Equivalent.
In addition, Son et al. stated that the content of polyphenols in the lettuce increased with the increasing blue light ratio. In our experiment BR light treatment increase the anthocyanin, phenolic compound and ascorbic acid in buckwheat plant support the previous findings [19]. Studies show that blue light is the most effective lighting source to synthesis anthocyanin by stimulating PAL (phenylalanine ammonia-lyase), CHS (chalcone synthesis) and DFR (dihydroflavonol-4-reductase) gene expression [27].
Molecular Genetic Characterization of Fusants from Protoplast Fusion of S. Cerevisiae and P. Stipitis ATCC 58785-https://biomedres01.blogspot.com/2020/11/molecular-genetic-characterization-of.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.