Reviving Previous Therapeutics by Recombinant Anaerobic Bifidobacteria
Introduction
Applications of Bacteria for Treatment Cancer
For the drug discovery and development, microorganisms have been indispensable as sources for screening seed compounds [1] and have been used to manufacture biopharmaceuticals through genetic engineering in the past decade [2]. Additionally, cancer immunotherapy, which has recently been examined in terms of immune-checkpoint inhibitors, has achieved cancer remission by activating human host immunity through bacterial infection [3]. In the 19th century, the American surgeon William B. Coley discovered the anti-cancer effect of erysipelas. He then performed cancer treatment with the heat-killed bacterial mixture known as Coley’s toxin [4], which induced regression of tumor tissues. This therapy is based on the release of immune suppression-related molecules in patients with cancer [5], and therefore causes pathological conditions such as fever or inflammation. Thus, the bacterial preparation should be administered to the topical tumor site or intratumorally. This concept has been applied to intravesical Bacillus Calmette-Guérin treatment for non-muscle invasive bladder cancer and has shown efficacy comparable to those of chemotherapies [6,7].
Applications of Bacteria for Treatment Cancer
For the drug discovery and development, microorganisms have been indispensable as sources for screening seed compounds [1] and have been used to manufacture biopharmaceuticals through genetic engineering in the past decade [2]. Additionally, cancer immunotherapy, which has recently been examined in terms of immune-checkpoint inhibitors, has achieved cancer remission by activating human host immunity through bacterial infection [3]. In the 19th century, the American surgeon William B. Coley discovered the anti-cancer effect of erysipelas. He then performed cancer treatment with the heat-killed bacterial mixture known as Coley’s toxin [4], which induced regression of tumor tissues. This therapy is based on the release of immune suppression-related molecules in patients with cancer [5], and therefore causes pathological conditions such as fever or inflammation. Thus, the bacterial preparation should be administered to the topical tumor site or intratumorally. This concept has been applied to intravesical Bacillus Calmette-Guérin treatment for non-muscle invasive bladder cancer and has shown efficacy comparable to those of chemotherapies [6,7].
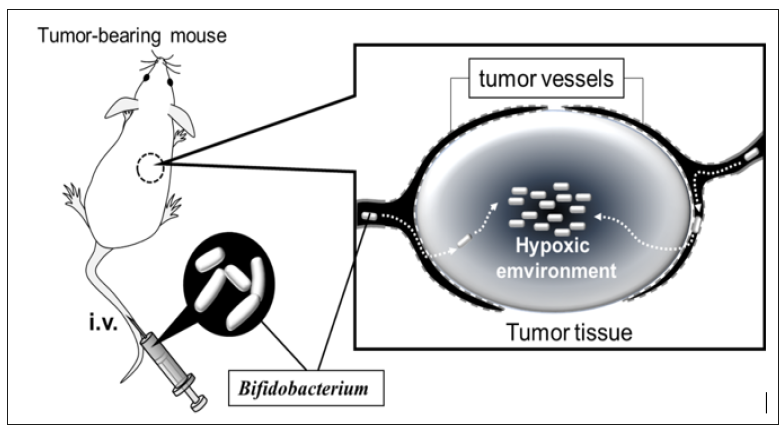
Anaerobic bacteria, including clostridia, S. Typhimurium, and L. monocytogenes, are distributed specifically into solid tumor tissues through enhanced permeability and retention effects [19]. Intravenously administered anaerobes invade tumor tissues from leaky tumoral blood vessels, after which they can survive and proliferate specifically in the intratumoral hypoxic environment [20] (Figure 1). Through progress in genetic engineering, these bacteria are considered as vectors for carrying anticancer proteins into tumor tissues [21-23]. Bifidobacteria are also wellknown obligate anaerobic bacteria that commonly inhabit the human and animal intestines. They are non-toxic gram-positive bacteria, and thus have no risk of endotoxin shock associated with their intravenous administration. Genetic modification has been difficult in most bifidobacterial cells [24]; however, gene transformation [25,26] and knock out [27] are practical methods in some bifidobacterial strains with high transformation efficiency [28]. Based on these advantages, recombinant bifidobacteria that express cytosine deaminase to convert prodrug 5-fluorcytosine into active 5-fluoruracil in tumor tissues have been developed [29].
 We established a system for delivering proteins into tumor tissue using recombinant bifidobacteria expressing physiologically active proteins. First, we aimed to re-examine the use of immunotoxins [30]. Immunotoxins did not show enough effectiveness during their clinical tests in the 1980s because of their systemic adverse effects and induction of neutralizing antibodies; thus, it is necessary to develop methods for their delivery into tumor tissues. To achieve this, we employed a toxin-releasing bifidobacteria as carrier and constructed the gene of pseudomonas exotoxin subunit A [31] lacking its receptor binding site (dPEA) to avoid invasion of toxin molecules into normal cells [32]. This subunit was fused with a bifidobacterial secretary peptide sequence and variable domain of heavy chain of heavy-chain antibody (VHH) gene of the camelid single-chain antibody [33] (Figure 2) against epidermal growth factor receptor (EGFR). EGFR is a well-known molecular target for cancer treatment [34]. EGFR is also suitable as an immunotoxin target because it is internalized in cells upon binding to ligands or agonists [35]. This VHH-dPEA gene construct was introduced into bifidobacterial cells, causing them to secrete VHH-dPEA fusion proteins and was named as the 8C7 toxin. The cloned 8C7 toxinsecreting bacteria exhibited anti-tumor effects when administered intravenously into tumor-bearing mice [36]. The anti-tumor effect of 8C7 toxin was comparable to that of gefitinib [36, 37] (Figure 3), and adverse effects such as weight loss or hepatic dysfunction were not observed.
We established a system for delivering proteins into tumor tissue using recombinant bifidobacteria expressing physiologically active proteins. First, we aimed to re-examine the use of immunotoxins [30]. Immunotoxins did not show enough effectiveness during their clinical tests in the 1980s because of their systemic adverse effects and induction of neutralizing antibodies; thus, it is necessary to develop methods for their delivery into tumor tissues. To achieve this, we employed a toxin-releasing bifidobacteria as carrier and constructed the gene of pseudomonas exotoxin subunit A [31] lacking its receptor binding site (dPEA) to avoid invasion of toxin molecules into normal cells [32]. This subunit was fused with a bifidobacterial secretary peptide sequence and variable domain of heavy chain of heavy-chain antibody (VHH) gene of the camelid single-chain antibody [33] (Figure 2) against epidermal growth factor receptor (EGFR). EGFR is a well-known molecular target for cancer treatment [34]. EGFR is also suitable as an immunotoxin target because it is internalized in cells upon binding to ligands or agonists [35]. This VHH-dPEA gene construct was introduced into bifidobacterial cells, causing them to secrete VHH-dPEA fusion proteins and was named as the 8C7 toxin. The cloned 8C7 toxinsecreting bacteria exhibited anti-tumor effects when administered intravenously into tumor-bearing mice [36]. The anti-tumor effect of 8C7 toxin was comparable to that of gefitinib [36, 37] (Figure 3), and adverse effects such as weight loss or hepatic dysfunction were not observed.Figure 2: Immunotoxin for the expression in bifidobacteria We utilized camelid VHH against EGFR to construct the immunotoxin. Unlike the variable region of a general antibody molecule composed of light and heavy chains, VHH derived from a camelid single-chain antibody can bind to an antigen as a single polypeptide. Because VHH is a very small molecule with excellent chemical stability, its application can lead to the development of pharmaceutical products.
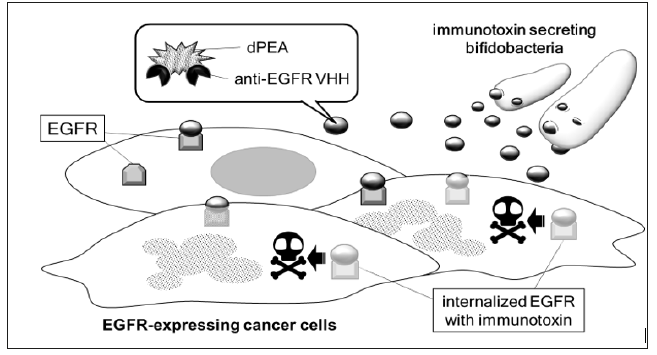
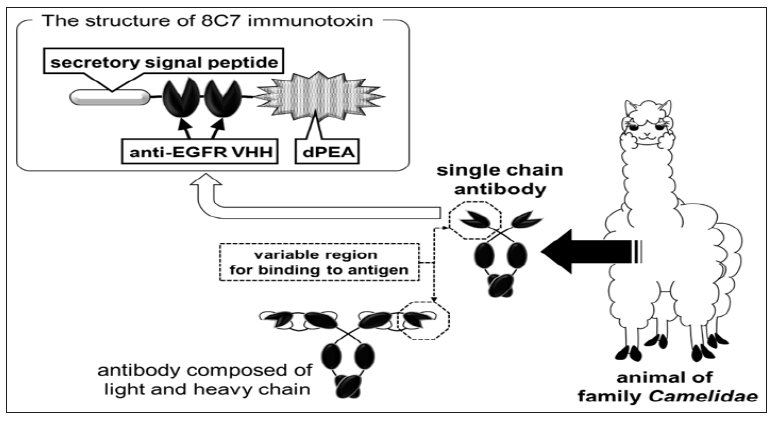 Figure 3: Schematic image of tumor cell-selective cytotoxicity by 8C7 immunotoxin Recombinant bifidobacteria administered directly into bloodstream accumulate selectively in tumor tissues and secrete 8C7 immunotoxin. 8C7 immunotoxin molecules bind to EGFR expressed on tumor cell surface, and then invade tumor cells by endocytosis. The dPEA portion of 8C7 immunotoxin causes cell death by inhibiting protein synthesis.
Figure 3: Schematic image of tumor cell-selective cytotoxicity by 8C7 immunotoxin Recombinant bifidobacteria administered directly into bloodstream accumulate selectively in tumor tissues and secrete 8C7 immunotoxin. 8C7 immunotoxin molecules bind to EGFR expressed on tumor cell surface, and then invade tumor cells by endocytosis. The dPEA portion of 8C7 immunotoxin causes cell death by inhibiting protein synthesis.
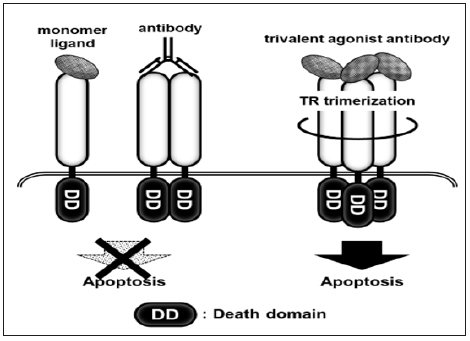
 We next targeted the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TR1 and TR2 are subtypes of the TRAIL receptor (TR) and transduce apoptotic signals [38,39]. There are at least six subtypes of TR, some of which act as decoy receptors. To circumvent decoy receptors, agonist antibodies against TR1 or TR2 were developed as anti-cancer agents. Unfortunately, these agonist antibodies based on immunoglobulins with two antigen-recognition sites showed low effectiveness, as TRs transduce apoptotic signals in a trimer form; thus, multivalent antibodies were developed (Figure 4). The multivalent antibodies exhibited high agonistic activity in preclinical tests, while in clinical trials, they caused sever hepatic dysfunction [40] because of the sensitivity of hepatic cells to TRAIL [41]. To establish safe and effective multivalent TR agonist antibodies, a drug delivery system specific to tumor tissue is required. We predicted that a bifidobacterial delivery system could be used to avoid the adverse effects of TR agonist antibodies and prepared recombinant bifidobacterial clones secreting trivalent TR1 or tetravalent TR2 agonistic antibodies with VHH [42]. These clones showed anti-tumor effects in tumor-bearing mice following intravenous administration, and critical adverse effects were not observed during the experiment.
We next targeted the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TR1 and TR2 are subtypes of the TRAIL receptor (TR) and transduce apoptotic signals [38,39]. There are at least six subtypes of TR, some of which act as decoy receptors. To circumvent decoy receptors, agonist antibodies against TR1 or TR2 were developed as anti-cancer agents. Unfortunately, these agonist antibodies based on immunoglobulins with two antigen-recognition sites showed low effectiveness, as TRs transduce apoptotic signals in a trimer form; thus, multivalent antibodies were developed (Figure 4). The multivalent antibodies exhibited high agonistic activity in preclinical tests, while in clinical trials, they caused sever hepatic dysfunction [40] because of the sensitivity of hepatic cells to TRAIL [41]. To establish safe and effective multivalent TR agonist antibodies, a drug delivery system specific to tumor tissue is required. We predicted that a bifidobacterial delivery system could be used to avoid the adverse effects of TR agonist antibodies and prepared recombinant bifidobacterial clones secreting trivalent TR1 or tetravalent TR2 agonistic antibodies with VHH [42]. These clones showed anti-tumor effects in tumor-bearing mice following intravenous administration, and critical adverse effects were not observed during the experiment.
 Figure 3: Schematic image of tumor cell-selective cytotoxicity by 8C7 immunotoxin Recombinant bifidobacteria administered directly into bloodstream accumulate selectively in tumor tissues and secrete 8C7 immunotoxin. 8C7 immunotoxin molecules bind to EGFR expressed on tumor cell surface, and then invade tumor cells by endocytosis. The dPEA portion of 8C7 immunotoxin causes cell death by inhibiting protein synthesis.
Figure 3: Schematic image of tumor cell-selective cytotoxicity by 8C7 immunotoxin Recombinant bifidobacteria administered directly into bloodstream accumulate selectively in tumor tissues and secrete 8C7 immunotoxin. 8C7 immunotoxin molecules bind to EGFR expressed on tumor cell surface, and then invade tumor cells by endocytosis. The dPEA portion of 8C7 immunotoxin causes cell death by inhibiting protein synthesis. We next targeted the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TR1 and TR2 are subtypes of the TRAIL receptor (TR) and transduce apoptotic signals [38,39]. There are at least six subtypes of TR, some of which act as decoy receptors. To circumvent decoy receptors, agonist antibodies against TR1 or TR2 were developed as anti-cancer agents. Unfortunately, these agonist antibodies based on immunoglobulins with two antigen-recognition sites showed low effectiveness, as TRs transduce apoptotic signals in a trimer form; thus, multivalent antibodies were developed (Figure 4). The multivalent antibodies exhibited high agonistic activity in preclinical tests, while in clinical trials, they caused sever hepatic dysfunction [40] because of the sensitivity of hepatic cells to TRAIL [41]. To establish safe and effective multivalent TR agonist antibodies, a drug delivery system specific to tumor tissue is required. We predicted that a bifidobacterial delivery system could be used to avoid the adverse effects of TR agonist antibodies and prepared recombinant bifidobacterial clones secreting trivalent TR1 or tetravalent TR2 agonistic antibodies with VHH [42]. These clones showed anti-tumor effects in tumor-bearing mice following intravenous administration, and critical adverse effects were not observed during the experiment.
We next targeted the receptor for tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). TR1 and TR2 are subtypes of the TRAIL receptor (TR) and transduce apoptotic signals [38,39]. There are at least six subtypes of TR, some of which act as decoy receptors. To circumvent decoy receptors, agonist antibodies against TR1 or TR2 were developed as anti-cancer agents. Unfortunately, these agonist antibodies based on immunoglobulins with two antigen-recognition sites showed low effectiveness, as TRs transduce apoptotic signals in a trimer form; thus, multivalent antibodies were developed (Figure 4). The multivalent antibodies exhibited high agonistic activity in preclinical tests, while in clinical trials, they caused sever hepatic dysfunction [40] because of the sensitivity of hepatic cells to TRAIL [41]. To establish safe and effective multivalent TR agonist antibodies, a drug delivery system specific to tumor tissue is required. We predicted that a bifidobacterial delivery system could be used to avoid the adverse effects of TR agonist antibodies and prepared recombinant bifidobacterial clones secreting trivalent TR1 or tetravalent TR2 agonistic antibodies with VHH [42]. These clones showed anti-tumor effects in tumor-bearing mice following intravenous administration, and critical adverse effects were not observed during the experiment.Figure 4: Induction of cell death by trimer TR agonist antibody When TRs bind with their ligands, they form into homo trimers and then induce apoptosis. Previous TR agonist products did not allow TRs trimerization because they were monomer ligands or agonist antibodies with two antigen-recognizing sites. In contrast, trivalent TR agonist antibodies simultaneously recognize three TRs in a single molecule, thus inducing cell death efficiently.
 Conclusion and Further Possibilities
Conclusion and Further Possibilities
As described above, the recombinant bifidobacteria are valuable as drug delivery carriers based on their selectivity for tumor tissues and safety. Additionally, their target molecules to fight tumor cells can be changed by varying their exogenous genes. Our system can be used to overcome the limitation of anticancer protein preparations discarded during their development because of their systemic side effects or poor tumor-specificity. Increases in pharmaceutical research and development (R&D) expenditures have become a serious problem among factors increasing medical expenses [43]. Additionally, the probability of successfully developing new medicines has decreased [44], and thus large numbers of pharmaceutical R&D projects are suspended or discontinued in preclinical or clinical trials.
 Conclusion and Further Possibilities
Conclusion and Further PossibilitiesAs described above, the recombinant bifidobacteria are valuable as drug delivery carriers based on their selectivity for tumor tissues and safety. Additionally, their target molecules to fight tumor cells can be changed by varying their exogenous genes. Our system can be used to overcome the limitation of anticancer protein preparations discarded during their development because of their systemic side effects or poor tumor-specificity. Increases in pharmaceutical research and development (R&D) expenditures have become a serious problem among factors increasing medical expenses [43]. Additionally, the probability of successfully developing new medicines has decreased [44], and thus large numbers of pharmaceutical R&D projects are suspended or discontinued in preclinical or clinical trials.
Some products developed through such projects were verified to be safe and effective, and thus have been converted into other new medicines by drug repositioning [45,46] or by combining technologies such as antibody-drug conjugation [47,48]. These drug-reuse approaches, including our bacterial product, are expected to reduce the costs and time required for drug development. The most conventional procedures for manufacturing protein-based biopharmaceuticals require large-scale systems for protein purification and production using animal cells and costly culture media. In contrast, our recombinant bifidobacteria are the protein producing system and can be administered to patient as bacteria themselves without protein purifying process. The massproduction of bifidobacteria can be achieved at a reasonable cost because they can grow in simple and low-cost medium. Therefore, a bifidobacterial delivery system substantially contribute to lowering medical expenditures both in the biopharmaceutical R&D process and manufacturing process.
Should we be 3D printing patient specific implants and using them?-https://biomedres01.blogspot.com/2020/11/should-we-be-3d-printing-patient.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.