Molecular Conformation Correlation to Activity Against Herpes Simplex Virus of (E)-5-(2-Bromovinyl)-2’-Deoxycytidine and 5-Methoxymethyl-2’-Deoxycytidine Analogs: Short-Review
Introduction
The antimetabolites (E)-5-(2-bromovinyl)-2’-deoxycytidine (BrVdCyd) (1) and 5-methoxymethyl-2’-deoxycytidine, MMdCyd (2) are selective and potent antiherpes agents with low cytotoxicity [1,2,3]. The antiviral activity of BrVdCyd (1) and MMdCyd (2) is influenced by the cytidine/deoxycytidine (Cyd/dCyd) deaminase and deoxycytidilate (dCMP) deaminase content of the cell lines used for antiviral assays [1]. When deamination is prevented, both (1) and (2) are potent inhibitors of herpes simplex virus type 1 (HSV-1). The IC99 of BrVdCyd (1) (concentration required to reduce the yield of infectious virus obtained 72 h after infection by 99% relative to control cultures) was 1.6 μM when (1) was used in combination with tetrahydro-deoxyuridine (H4dUrd; an inhibitor of both dCyd and dCMP deaminases) (1). BrVdCyd (1) is also a good inhibitor of varicella zoster virus (VZV) replication [1]. Previous studies have shown that resistance to deamination for cytidines can be accomplished by structural modifications of the cytidine molecule [3-8]. Therefore, systematic investigations on the development of 5-substituted deoxycytidine analogs of (1) and (2) resistant to deamination were initiated.
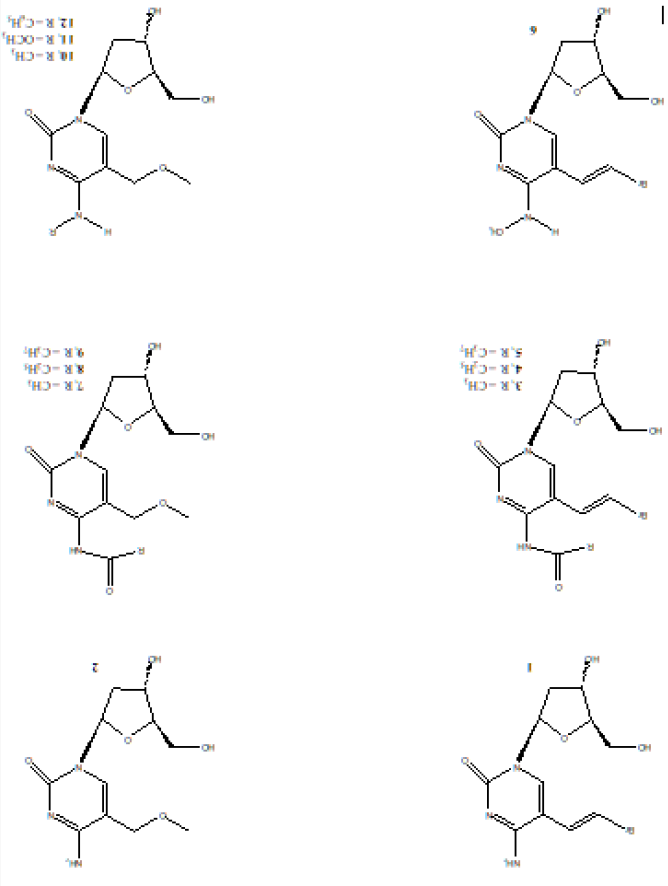
The rationale is that deoxycytidine analogs resistant to deamination would retain selectivity and metabolic stability thus simplifying HSV-1 treatment regimens. Therefore, N4-substituted derivatives of (E)-5-(2-bromovinyl)-2’-deoxycytidine (BrVdCyd) and 5-methoxymethyl-2’-deoxycytidine (MMdCyd) were synthesized to confer resistance to deamination, to improve delivery of the antimetabolite and to determine the bulk tolerance of the viral kinase to the N4-substituents of the cytosine moiety. The molecular conformation in solution was determined by NMR spectroscopy and the activity against herpes simplex virus type 1 (HSV-1) was determined using A549 cells. Compounds (3) and (4) were also found to be 100 times more potent and selective inhibitors of the varicella zoster virus (VZV) compared to the widely used antiviral drug acyclovir [9]. Chemical structures for compounds (1 - 12) are shown in Figure 1.
Figure 1: Chemical structures of compounds (1 – 12). BrVdCyd (1), MMdCyd (2), N4-acetyl-BrVdCyd (3), N4-propanoyl- BrVdCyd (4), N4-butanoyl-BrVdCyd (5), N4-methyl-BrVdCyd (6), N4-acetyl-MMdCyd (7), N4-propanoyl-MMdCyd (8), N4- butanoyl-MMdCyd (9), N4-methyl-MMdCyd (10), N4-methoxy-MMdCyd (11), N4-phenyl-MMdCyd (12).
Experimental
Antiviral Activity
The effectiveness of compounds (3-12) as inhibitors of HSV- 1 (McIntyre strain) replication was determined by the plaque reduction assay using A549 cells. The parent compounds BrVdCyd (1) and MMdCyd (2) were used as a positive control. N4-Acetyl- BrVdCyd (3) was significantly more active (ED50 = 0.01 μM) than its parent compound BrVdCyd (1) (ED50 = 0.61 μM), N4- propanoyl-BrVdCyd (3) was a good inhibitor of HSV-1 replication (ED50 = 0.12 μM) and N4-butanoyl-BrVdCyd (4) (ED50 = 6.00 μM) was a poor inhibitor compared to their parent compound (1). N4- Methyl-BrVdCyd (5) was devoid of activity (ED50 > 1024 μM). All compounds have low cytotoxicity and can be considered essentially nontoxic as measured by the CC50 (cytotoxic concentration required to reduce cell growth by 50% using confluent monolayers of A549 cells). Table 1 shows the antiviral activity data for all compounds with acyclovir’s activity added for comparison.
Table 1: Antiviral activity of the compounds listed against HSV type-1 in A-549 cell line1.
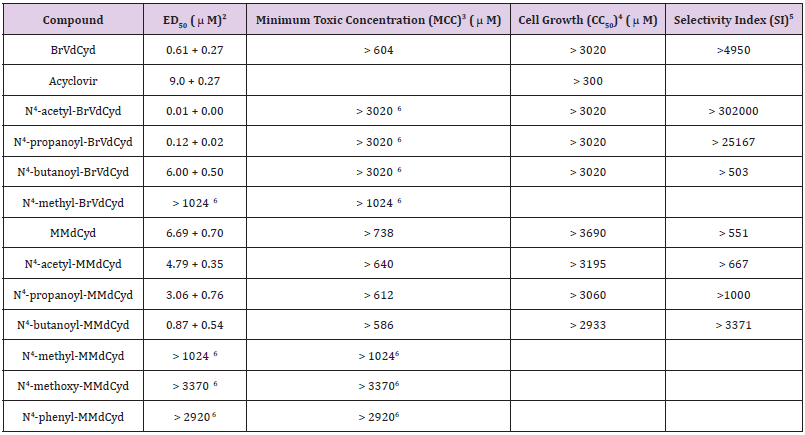
Note:
a) HSV-1 McIntyre strain was used. Virus input was 50 PFU.
b) ED50: Inhibitory concentration required to reduce viral plaques by 50% (mean + SD, n = 12)
c) MCC: Minimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology.
d) CC50: Cytotoxic concentration required to reduce cell growth by 50%.
e) SI: Ratio of CC50 /ED50.
f) Highest concentration tested.
Conformational Analysis
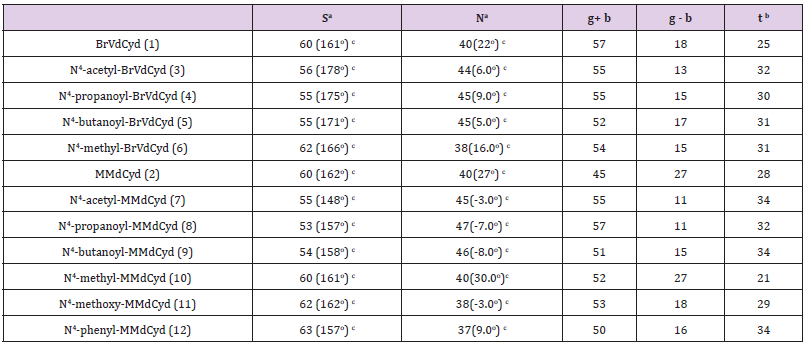
The in-solution conformation of compounds (1-12) was determined by NMR spectroscopy using D2O as a solvent. Four different conformational aspects were under consideration (Figure 2): The North/South deoxyribose ring pseudo-rotational conformation, the Syn/Anti glycosidic conformation, the exocyclic side-chain conformation (g+, g- and t) and finally orientation of the N4-substituent (proximal to C5 or proximal to N3 of the pyrimidine ring). The proton-proton coupling constants are given in Table 2; conformational parameters are summarized in Tables 3 & 4. The four conformational modes under study are shown in Figure 2 (structures for N4-substituted BrVdCyd and its analogs are provided). The conformation of the deoxyribose sugar ring was obtained form the relationship between the proton-proton coupling constants and the pseudo-rotational properties (Table 3) of the ring using the computer program PSEUROT [10]. The population of the three rotamers (g+, g- and t) about the exocyclic C(4’)-C(5’) bond (Table 3) was determined from the J4’5’ and J4’5’’ coupling constants [11,12].
Figure 2: The four conformational modes in pyrimidine deoxyribonucleoside analogs discussed in this study.
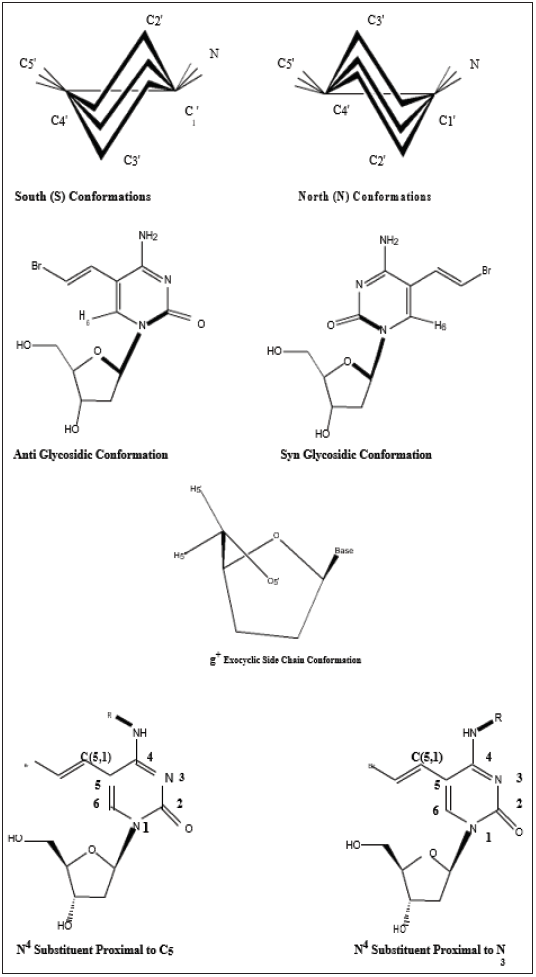
Table 2: Vicinal coupling constants (experimental and calculated in Hz) of BrVdCyd (1), N4-acetyl-BrVdCyd (3), N4-propanoyl- BrVdCyd (4), N4-butanoyl-BrVdCyd (5), N4-methyl-BrVdCyd (6), MMdCyd (2), N4-acetyl-MMdCyd (7), N4-propanoyl-MMdCyd (8), N4-butanoyl-MMdCyd (9), N4-methyl-MMdCyd (10), N4-methoxy-MMdCyd (11) and N4-phenyl-MMdCyd (12)
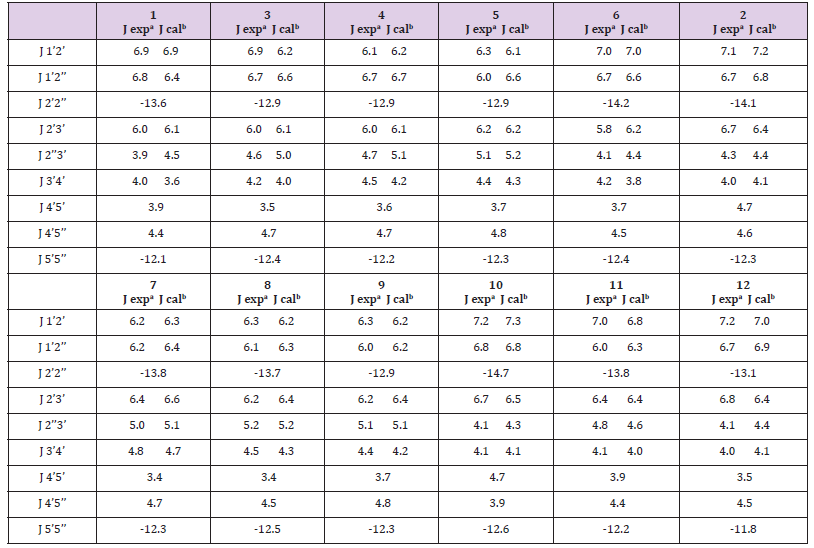
a: J experimental; precison 0.1 Hz.
b: J calculated using PSEUROT with tm =360 C
Table 3: Conformation populations (%): South (S) and North (N) furanose ring conformers and the three rotamers (g+, g- and t) of the exocyclic C(5’) side chain of BrVdCyd, MMdCyd and their N4 substituted analogs.
Note:
a) In PSEUROT calculations, μm was constrained to 36.0o. The rms deviation for each calculation was: BrVdCyd: 0.193; N4- acetyl-BrVdCyd: 0.250 E; N4-propanoyl-BrVdCyd: 0.227 E; N4- butanoyl-BrVdCyd: 0.179 E; N4-methyl-BrVdCyd: 0.175 E; N4- acetyl-MMdCyd: 0.204 E; N4-propanoyl-MMdCyd: 0.166 E;N4-butanoyl-MMdCyd: 0.181 E; N4-methyl-MMdCyd: 0.112 E; N4- methoxy-MMdCyd: 0.145 E; N4-phenyl-MMdCyd: 0.202 E.
b) C5’ Exocyclic orientation at 37oC.
c) Numbers in brackets are the calculated pseudo-rotational phase angles PS and PN
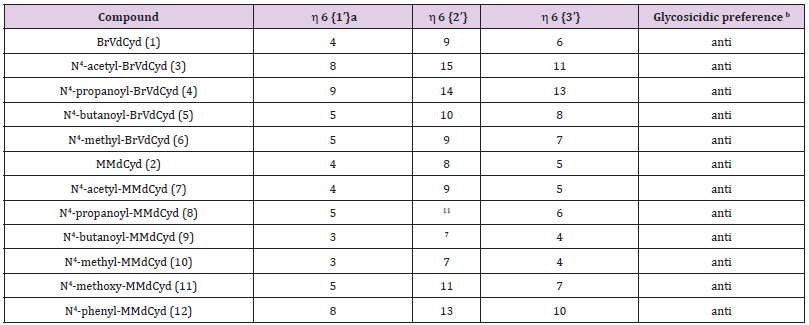
The syn/anti glycosidic preference (orientation of the pyrimidine ring relative to the deoxyribose moiety) defined by the torsion angle O-C(1’)-N(1)-C(2) was determined by nuclear overhauser enhancement (nOe), the enhancement of deoxyribose protons H1’, H2’ and H3’ were observed on pyrimidine H6 using the method of Davies [13] and the data are summarized in Table 4. The position of the N4-substituents (proximal to C (5) or proximal to N (3)) was elucidated by observing the nOe of the N4-substituent protons or lack of it on C (5,1) and C (5,2) olefinic (vinyl) protons in BrVdCyd analogs and in the case of MMdCyd analogs, the nOe of the N4-substituent protons or lack of it on C (5,1) methylene and C (5,3) methoxy protons [14].
Table 4:Syn/anti glycosidic preference of BrVdCyd, MMdCyd and their N4 substituted analogs.
Note:
a) The common notation used for nOe is ηi {s} which indicates that this is the nOe of nucleus i when nucleus {s} is saturated.
b) Glycosidic preference refers to a dynamic solution equilibrium that is biased towards either syn or anti. For syn orientations, H6 is closest to H1’ and the nOe to H6 will be mainly from H1’. For anti-orientations, the nOe to H6 will be mainly from H2’ and H3’
Results and Discussion
Deoxyribose Ring Conformation
The four major modes of conformational flexibility for pyrimidine 2’-deoxyribonucleosides are shown in Figure 2. The conformation of the deoxyribose moiety is important in determining the biological activity of 5-substituted 2’-deoxyribonucleosides against herpes simplex virus and varicella zoster virus [2,3,5,15]. All compounds (1 - 12) displayed South/North equilibrium for the deoxyribose conformation in the range from 53/47 to 63/37.
C (5’) Exocyclic Side Chain Conformation
Molecular conformation studies by X-ray crystallography (solid state) and NMR spectroscopy (in D2O) indicate that the conformation of the 5’-exocyclic side chain [γ torsion angle C (3’)- C (4’)-C (5’)-O (5’)] is important in determining the activation of 5-substituted pyrimidine-2’-deoxynucleosides by HSV- induced thymidine kinase (HSV-TK). The g+ conformer seems to be the preferred orientation required by HSV-TK; whereas the t conformer appears to be an unfavored conformation [3,7,9,15]. All compounds (1 - 12) have a predominant g+ rotamer for the C (5’) exocyclic side chain (Table 3).
Syn/Anti Glycosidic Conformation
The syn/anti glycosidic equilibrium in all compounds (1-12) is biased towards the anti-region (Table 4).
N4-Substituent Orientation
The in-solution orientation of the N4-acyl substituent in anti HSV-1 active compounds of both series BrVdCyd (1) and MMdCyd (2) (compounds 3, 4, 5, 7, 8 and 9) is proximal to pyrimidine C (5). Whereas the orientation of the N4 substituent in compounds 6, 10, 11 and 12 all of which showed no anti HSV-1 activity is proximal to pyrimidine N (3). The aforementioned results were established by nOe experiments on all ten derivatives of BrVdCyd (1) and MMdCyd (2) namely compounds 3 - 12.
Conclusion
In considering the four conformational modes of the compounds under study, we found that those analogs which had no activity against HSV-1 have three of the four modes in common with those analogs which were active against HSV-1, namely the deoxyribose ring conformation (predominantly south), the exocyclic sidechain (predominantly g+) and the glycosidic bond conformation (predominantly anti). The one difference between analogs which are active against HSV-1 and analogs which are not is the N4- substituent orientation. Anti-HSV-1 active analogs had the N4- substituent oriented proximal to pyrimidine C (5) whereas analogs lacking any activity against HSV-1 had the N4-substituent oriented proximal to pyrimidine N (3). Therefore, it is safe to conclude that an N4-substituent oriented proximal to pyrimidine N (3) prevents in some way an analog from exhibiting activity against HSV-1. It is obvious that the potency trends in the two series N4-acyl-BrVdCyd and N4-acyl-MMdCyd analogs run in opposite directions. While in the one series, the analog N4-acetyl-BrVdCyd is the most active among three N4-acyl analogs, it is the N4-butanoyl-MMdCyd that is most active among its three N4-acyl analogs.
In general, we conclude that the N4-acyl substituents conferred resistance to deamination in MMdCyd analogs with N4-butanoyl- MMdCyd being the slowest to undergo hydrolysis of the N4- butanoyl group and eventual deamination, hence shows the greatest antiviral activity. Steric bulk in N4-acyl MMdCyd substituents did not seem to affect ease or rate of phosphorylation by HSV-1 induced thymidine kinase. We also conclude that N4-acyl substituents conferred resistance to deamination in BrVdCyd analogs yet it appears that bulk tolerance reverses the anti HSV-1 trend seen earlier in MMdCyd analogs. N4-Acetyl-BrVdCyd shows resistance to deamination and an overall tolerable bulk. As the substituent grows bulkier to N4-propanoyl and N4-butanoyl, anti HSV-1 activity is less especially with N4-butanoyl-BrVdCyd perhaps due to docking and rate of phosphorylation by HSV-1 induced thymidine kinase.
Presumed Orbital Infarction Syndrome after Functional Endoscopic Sinus Surgery (FESS)-https://biomedres01.blogspot.com/2021/01/presumed-orbital-infarction-syndrome.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.