Organophosphate Pesticide Exposure and Glucose Homeostasis
Introduction
Organophosphate (OP) compounds have been used as pesticides since 1975. OP insecticide compounds are a diverse group of chemicals. This compound contains different group of chemicals such as acephate, diazinon, dimethorate, parathion, phosmet, malathion, parathion, diazinon, fenthion, dichlorvos, chlorpyrifos, ethion, azamethiphos [1]. There is emerging evidence that OP has various toxicities such as neurotoxicity, endocrine toxicity, immunotoxicity, reproductive toxicity, genotoxicity and cellular oxidative imbalance and impaired glucose homeostasis at the hazard of human and animal health. It was found the notable coincidence of occurrences in association between OP poisoning and severe hyperglycemia. Two patients, mother and son presented with many complicated symptoms including hyperglycemia and administration of pralidoxime (AChE activator) normalized blood glucose level. Though acetylcholinesterase enzyme level had not been detected in these patients, they had history of exposure to malathion sprayed in their area [2]. Many case report studies had described the detail sign and symptoms of OP pesticide poisoning accompanying hyperglycemia. Then , it has been raised awareness of association between OP poisoning and severe hyperglycemia in human.
Effect of OP Pesticide on Liver Enzymes Involved in Glucose Homeostasis Pathways
Culminating hyperglycemia has been increasingly reported as outcome of OP poisoned animal model studies. Exposure of rats to OP compounds such as Malathion [3-5], acephate [6] and dimethoate [7] developed hyperglycemia with stimulation of hepatic gluconeogenesis and glycogenolysis. The possible mechanism of increase blood glucose level might be due to stimulation of hepatic gluconeogenesis and glycogenolysis for increased energy production to detoxification. Upon entering the body– through contact with skin and mucous membrane, inhalation and ingestion– OP pesticides avidly binds the AChE by forming covalent bond between OP pesticides and oxygen of serine at the active site of AChE then it transforms into irreversible phosphorylated inactive AChE and lead to increased acetylcholine activity [8,9]. Thus, hyperesthesia, intermittent spasm, muscular tremors, sustained seizures and muscle fasciculation are induced by cholinergic action of OP pesticides [10]. These involuntary energy demanding activities trigger the release of glucose by glycogenolysis and gluconeogenesis. It also stimulates the glycolysis of liver and muscle, and subsequent releases of ATP to meet the body’s energy requirement [4,11].
Effect of OP Pesticide Exposure on Oxidative Stress
Exposure to OP pesticides, inhibits the AChE enzyme and increases the activity of muscarinic type of Ach receptor. The overstimulation of Ach receptors result in uncoordinated nerve and muscle stimulation leading to sustained seizures and muscle fasciculation [10]. During these conditions, the flow of oxygen through brain and muscle is greatly increased. This metabolic stress results in increase requirement the ATPs for them and increase demand of glucose, which in turn activates the glycolysis. Glycolysis is oxygen independent metabolic pathway that converts glucose into pyruvate. This process releases the high energy molecule ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide) [12,13]. These products of glycolysis are oxidized by using atmospheric oxygen.
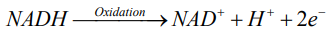
According to redox reaction, oxidation is a process in which an atom or a group of atoms taking part in chemical reaction loses one or more electrons and remain an uneven number of electrons. An atom or group of atom with an even number of electron (unpaired electron) is also known as free radical (ROS). Rapid release of ROS due to increase metabolism is called respiratory burst. Respiratory burst activates cell membrane-bound enzyme NADPH oxidase (NOX) which catalyzes one-electron reduction of oxygen to superoxide anion, and the process involves oxidization of cytosolic NADPH to NADP and free radicle (ROS).
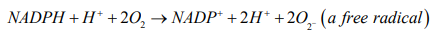
Oxidative Stress and Insulin Resistance
Insulin is the major hormone controlling glucose metabolism. When the blood glucose level is increased, glucose stimulates insulin secretion from beta cell of pancreas. When glucose enters the beta cell, glucose is metabolized, and ATP was generated. Then, closure of ATP sensitive K+ channel increases intracellular K+ concentration and cause the membrane depolarization. And then opening of voltage-gated Ca2+ channels open and increased Ca2+ concentration in cytoplasm causes activation of protein kinase and release of insulin by exocytosis [14,15]. When insulin binds to insulin receptor α subunits, there is autophosphorylation of β subunits on tyrosine residues and activates the insulin receptor tyrosine kinase, which phosphorylates and recruits different substrate adaptors such as the Insulin receptor substrate (IRS) family of proteins. Tyrosine phosphorylated IRS, then displays binding sites for phosphatidylinositol 3- kinase (PI3-kinase). PI3- kinase then phosphorylates phosphatidylinositol 2- kinase (PIP2) to PIP3 which in turn activate protein kinase B (Akt), eventually leading to glucose transporter 4 (GLUT4) translocation to plasma membrane of skeletal muscle cells and adipocyte, thus allowing the cells to absorb extracellular glucose, lowering plasma glucose level.
Ma et al. proposed the mechanism about the oxidative stress and insulin resistance [16]. During the oxidative stress, NADPH oxidase 4 (NOX4) is a powerful oxidizing enzyme that produces ROS. In this condition, there was a shift in the signaling pathway that happens at PI3-kinase. PI3-kinase phosphorylates Rac GTP ase instead of PIP2, which amplifies the activity of NOX4 [17]. The increased ROS activates casein kinase-2 (CK2) which in turn activates the retromer. The retromer then signals the trans-Golgi network downstream, and GLUT4 is transported to lysosomes for degradation instead of the plasma membrane. Therefore, intravascular glucose levels remain elevated and cause the insulin resistance.
Conclusion
The anticholinesterase effects of OP insecticides are produced by the binding of the phosphate groups of this chemicals compound to the AchE that hydrolyzes acetylcholine (Ach) neurotransmitter at the neuromuscular junction. Excessive accumulation of Ach at cholinergic site produce nicotinic effects including intermittent spasm, muscular tremors, sustained seizures and muscle fasciculation resulting from prolonge continuous stimulation of Ach neurotransmitter at motor endplate. Thus, oxygen and glucose demand as well as ATP requirement are greatly increased throughout the muscles. After that, the glycolysis pathway is activated to achieve the increase energy requirement and NADH as a product of glycolysis pathway is oxidized by using atmospheric oxygen resulting in increased production of free radical (ROS). These ROS shift the insulin signaling pathway at PI3-kinase to phosphorylate the Rac GTPase leading to degradation of the GLUT4 in lysosome. Thus, intravascular glucose levels remain elevated and cause the insulin resistance.
Risk Factors and Prevention of Venous Thromboembolism in Ambulatory Cancer Patients Requiring Chemotherapy-https://biomedres01.blogspot.com/2021/02/risk-factors-and-prevention-of-venous.html
More BJSTR Articles : https://biomedres01.blogspot.com



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.