Efficacy of Sorafenib in Patients with Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization
Introduction
Hepatocellular carcinoma (HCC) is the third most common cause of cancer-related death worldwide, with an escalating global incidence and mortality [1]. In Japan, nationwide follow-up surveys by the Liver Cancer Study Group of Japan revealed that the total number of deaths from liver cancer increased steadily in 1975 but have been declining since its peak in 2004. The establishment of a nationwide HCC surveillance program in Japan has contributed to the improvement of overall survival (OS). Transcatheter arterial chemoembolization (TACE) is indicated as the first-line treatment for patients with intermediate-stage HCC [2]. On the contrary, sorafenib, which demonstrated survival benefit in the SHARP trial/Asia-Pacific trial, is applied for cases with advanced stage of HCC [3-6]. For example, sorafenib is indicated for cases with extrahepatic metastasis and vascular invasion. However, HCC patient’s refractory to TACE should also be treated with sorafenib before the progression to advanced stage because continuous TACE may lead to deterioration of liver function. From this point of view, the timing of switching treatment from TACE to sorafenib is critical for the management of HCC [7]. This study aimed to address this important issue and compare OS from the initial TACE between patients who continued TACE and those switched to sorafenib after refractoriness to TACE was observed.
Materials and Methods
Patients
The derivation dataset included 497 patients who underwent TACE for HCC at Kindai University between 2008 and 2013. All patients satisfied the diagnostic criteria for HCC proposed by the Japan Society of Hepatology (JSH) [8]. The diagnosis of HCC was made based on histology or radiologic findings using contrast-enhanced computed tomography (CE-CT) and/or dynamic magnetic resonance imaging (MRI). The selection of treatment was based on discussions involving multidisciplinary teams at our institution. Among these patients, intermediate-stage HCC patients who underwent multiple TACE sessions were retrospectively identified from medical records. Patients deemed refractory to TACE were further identified among the patients treated with repeated TACE. Then, these TACE-refractory patients were subdivided into two cohorts:
a. patients who switched from TACE to sorafenib (sorafenib group) or
b. patients continued TACE despite the suspicion of being refractory to TACE (TACE group).
The inclusion criteria were as follows:
a) patients with HCC diagnosed based on histological examination or typical radiological findings (early enhancement followed by late wash-out on CE-CT or dynamic MRI) and HCC refractory to radiofrequency ablation and surgical resection based on the indications for TACE,
b) Barcelona Clinic Liver Cancer (BCLC) stage B (intermediate stage),
c) performance status of 0 or 1, and
d) Child-Pugh class A or B liver function.
The exclusion criteria were
a. concomitant antineoplastic treatment,
b. BCLC stage C (advanced stage), and
c. history of hepatic arterial infusion chemotherapy/ systemic therapy after being refractory to TACE.
Clinicopathological variables including demographics, alphafetoprotein (AFP), Child-Pugh class, and BCLC prognostic scores were collected at the time of referral to our unit, prior to treatment
Definition of TACE Refractoriness
CE-CT is performed within 3 months of the TACE procedure to evaluate the radiological response of the tumor. Follow-up CT or MRI is performed every 3–4 months. In addition, AFP levels are evaluated every 2–3 months. The definition of TACE refractoriness is based on the JSH Consensus Guidelines [9]. The radiological response to TACE was evaluated on initial CT/MRI within 3 months after the latest TACE. The response of serum tumor marker levels was also evaluated after 2 months of the latest TACE. The increase in serum AFP level at >20% from baseline, within 2 months after the latest TACE, was considered continuous elevation of AFP level. For the assessment of response based on the AFP level, only patients with baseline AFP of >20 ng/ml were included.
Statistical Analysis
Survival curves were estimated using the Kaplan-Meier method with the primary endpoint of death for OS analysis from initial TACE. Patients who did not meet the endpoint were censored at the time of the last visit. Comparison of survival periods among the groups was estimated using the log-rank test. A P value of <0.05 was considered statistically significant. All analyses were performed using SAS statistical software version 8.2 (SAS Institute, Cary, NC, USA).
Results
Patient Characteristics
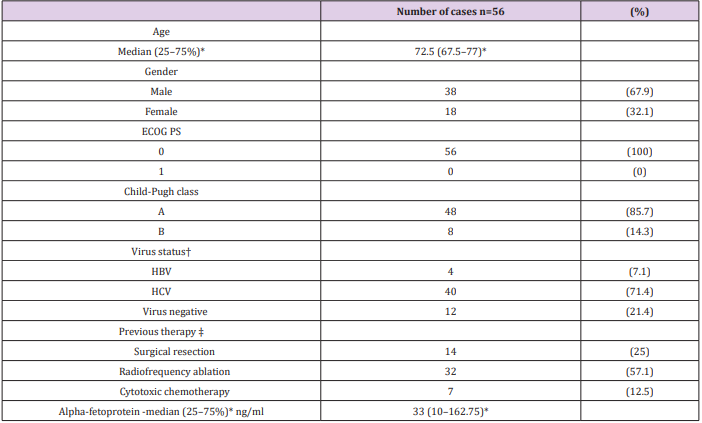
Among 497 patients who received TACE, 56 patients were considered refractory to TACE. The baseline characteristics of patients are summarized in Table 1. Forty patients (71.4%) were positive for anti-hepatitis C virus antibody (HCV Ab), four patients (7.1%) were positive for hepatitis B virus (HBV) surface antigen (HBs-Ag), and 12 patients (21.4%) were negative for both HCV Ab and HBsAg. All patients were asymptomatic with a performance status of 0. Forty-eight patients (85.7%) were classified as Child-Pugh class A.
Table 1: Baseline patient and disease characteristics.
ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; PS, performance status
*Dispersion variables are shown as median values (25-75%)
†Cases positive for hepatitis B virus surface antigen were regarded as cases of HBV-related HCC and cases positive for hepatitis C antibody were regarded as cases of HCV-related HCC
‡Patients may have received more than one type of therapy.
Treatment After TACE Refractoriness
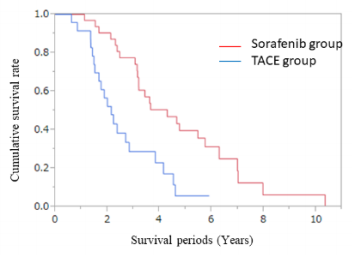
Of the patients deemed refractory to TACE, 32 patients were classified in the sorafenib group and 24 patients in the TACE group. Of the 56 patients (32 in the sorafenib group and 24 in the TACE group), 38 died during the study period (19 in the sorafenib group and 19 in the TACE group), 8 survived (5 in the sorafenib group and 3 in the TACE group), and 10 were lost to follow-up (8 in the sorafenib group and 2 in the TACE group). The median OS from the initial TACE of the entire cohort was 3.0 years (95% confidence interval (CI) 2.30–3.61 years). The median OS from the initial TACE was 2.14 years (95 % CI 1.51–2.83 years) and 4.29 years (95 % CI 3.15–5.74 years) for the patients in the TACE and sorafenib groups, respectively. Pairwise comparisons verified a significantly longer OS for patients in the sorafenib group than those in the TACE group (P < 0.001, log-rank test) (Figure 1).
Figure 1: Comparison of overall survival between transcatheter arterial chemoembolization and sorafenib in patients who were refractory to TACE. Kaplan-Meier curves of the overall survival of 56 patients who underwent treatment with continuous TACE or conversion to sorafenib after being considered refractory to TACE. The median overall survival of the patients in the TACE and sorafenib groups was 2.14 and 4.29 years, respectively, after being thought refractory to TACE (P < 0.001 by logrank test).
Discussion
Ogasawara et al. compared the efficacy of sorafenib treatment and continuation of TACE in HCC patients after being refractory to TACE and reported that OS and time to disease progression were longer in patients who switched from TACE to sorafenib than in those who received continuous TACE [10]. Our study also confirmed that after refractoriness to TACE was observed, OS could be improved by switching to sorafenib among the patients with intermediatestage HCC and TACE refractoriness. This study demonstrated that OS from the initial TACE was longer in the sorafenib group than that in the TACE group among TACE-refractory HCC patients. Although TACE is effective in cases with multiple nodules, repeated TACE could result in the obstruction of tumor vessels [11]. In addition, tumor progression may lead to incomplete embolization because of the invasive tumor characteristics without fibrous capsule. Because the antitumor effect of TACE should be counterbalanced by the deterioration of liver function, which is one of the adverse events of TACE, continuation of TACE in refractory cases should be avoided. Conversely, recent progress in HCC treatment, including molecular targeted agents, allows us to select various treatments: the degree of liver function has generally been considered in the selection of treatment in HCC cases [12-16]. Furthermore, in addition to the conventional HCC treatments, several clinical trials are being conducted such as on immune checkpoint inhibitor for HCC; it is quite possible that TACE would be replaced by systemic therapy in considerable number of HCC cases with intermediate stage [17-19]. Although TACE is indicated for patients with intermediate-stage HCC under the current algorithm, tyrosine kinase inhibitors, such as sorafenib or lemvatinib, is selected in many cases that demonstrate refractoriness to TACE.
Conclusion
In conclusion, it is likely that switching to sorafenib improves OS from initial TACE in TACE-refractory patients with intermediatestage HCC; chemoembolization should not be repeated if tumors are uncontrolled by previous TACE. To improve the survival of HCC patients, it is important to switch treatment from TACE to systemic therapy, such as sorafenib, in cases that demonstrate refractoriness to TACE even if HCC is still in the intermediate stage.
More BJSTR Articles : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.