Immobilization of Human Sperms by Gossypol Incorporated into l-Ascorbyl Palmitate Coagel
Introduction
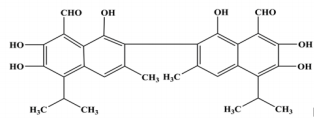
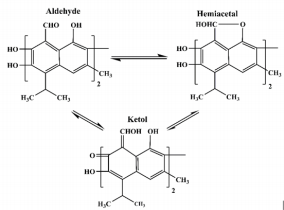
Gossypol, a natural complex polyphenolic extract (Figure 1) from cotton plants (Gossypium ssp.); it is a highly reactive organic compound due to its carbonyl and polar groups (6 hydroxyls and 2 aldehydes) as well as to its bulky binaphtalene structure [1]. Its phenolic groups readily react to form esters and ethers; whereas the aldehyde groups react with amines to form Schiff’s bases and with organic acids to form heat labile compounds or it may either also react with other products of the plant to form bound gossypol or remains as free gossypol which is attributed to be its most toxic form [2,3] through the clarifications of the multiple reactivity of gossypol, it is proposed that gossypol exists in three tautomeric (aldehyde, ketonoid, and the hemiacetal) forms (Figure 2) through which it may interchange into many different reactive sites [4]. Therefore, through many researches concerning the polyvalent reactivity of gossypol and its derivatives; it was interestingly discovered its versatile biological activities including: anticancer [5- 7]; antioxidant [8]; antimicrobial against main human pathogenic microorganisms such as bacteria, fungi, protozoa and yeasts [9]; antiviral activities against herpes simplex virus [10] human immunodeficiency virus-1 [11-12], some arboviruses and influenza type viruses; insecticidal [13-15] and antifertility activities [16-18].
In fact, even though gossypol exerts such potential biological activities ; its inescapable systemic toxicity is still a big limit from its clinical uses per any usual route of administration; gossypol has a high binding affinity with albumin and iron of hemoglobin of red blood cells thereby forming a strong complex leading to generalized anemia, lungs edema or hemorrhages and respiratory distress, mostly death depends on ingested amount and time of exposure [19-21]; gossypol acts as Prostaglandins biosynthesis stimulating agent and Na-K-ATPase inhibitor leading to renal potassium loss thereby causing chronic hypokalemia-causing cycle in some subjects [22,23]; it was also reported to be associated with reduction in number and early degeneration of testicular leyding cells thereby probably causing drug-induced sterility through negative interferences with spermatogenesis cycle [24-26]. Therefore, the systemic toxicity of gossypol and its derivatives is mainly species and dose-dependent but also time of exposure and route of administration may play an important role.
Through some series of clinical trials, no side effects were enhanced by small doses, even before humans have used gossypolcontaining drugs (bronchitis and cough) and foods but few and negligible related adverse effects were reported [27-29], the general accepted standards of gossypol consumption have been set to be no more than 0.8mg/kg/day for 6weeks as the maximum safety dose human species can tolerate [30,31].
Lately, after understanding the potentials of gossypol and its derivatives and after studying the mechanisms of action of severity of its adverse effects; many studies have been conducted trying to counteract its toxicity and maintaining its pharmacological activities either by co-administration with selenium and potassium supplements or by suppressing aldehyde groups on which lies its toxicity [32-34]. In the framework of minimizing hematological side effects caused by gossypol toxicity; the development of gossypol gel was successful achieved and reported neither side effects nor skin irritations on monkeys and female humans as an effective spermicidal contraceptive in vitro and in vivo [35-37]; this was a promising step proving that gossypol used topically is safer than other usual routes of administration. In this way; our laboratory successfully produced two kinds of liposoluble topical formulations by incorporating gossypol into l-ascorbyl ibuprofenate (l-AI) coagel [38] and incorporating gossypol into l-ascorbyl palmitate (l-AP) coagel [39].
Recently, the studies of rheological and viscoelastic properties of these coagels were conducted and revealed that these formulations are the best carriers of hydrophobic drugs like gossypol through the skin and they exert a limited gossypol release either from the drug formulation or skin reservoir systems through the skin but without passing deeper through stratum corneum to the system circulation and it was found that gossypol released from the coagel formulation remained entrapped on and within the outermost skin layer where reside most human pathogenic microorganisms before their distribution into systemic living systems [40] and this is a proof of a trustworthy topical dug formulation promising to restrict the systemic toxicity of gossypol once used in the future as a new and effective drug having potential abilities of acting on most human pathogenic microorganisms and spermicide. The evaluation of this gossypol-containing formulation refers to the facts that gossypol was previously reported to have not only contraceptive properties of acting as the most powerful male antifertility agent possibly effective at 99.9% [41] and sperms motility barrier, spermicidal [42-44] and spermatogenesis inhibitor [44], inhibitor of ovum implantation and early pregnancy [45-46] in different animals and human both in vitro and in vivo studies but, also its ability to prevent some sexual transmitted infections (STIs) based on previously reported polyvalent biological activities of gossypol and its derivatives [47-49].
Materials and Methods
Incorporation of gossypol into l-ascorbyl palmitate (l-AP) coagel started by dissolving l-ascorbyl palmitate powder (purchased from Hebei Xing Run Biological Technology Co. Ltd.) to make 4% l-AP coagel and successfully achieved by dissolving gossypol powder into l-AP coagel as previously described [26] with controlled mixing and temperature to make 0.1%, 0.2% 0.3%, 0.4% and 0.5% (w/w) gossypol-containing coagel formulations, a small concentration of glycerol less than 3% (v/v) was also used during coagel formulation as recommended in previous studies [50-52], immobilization activity was assessed following modified DP. Waller method [53]. The statistical descriptive data were analyzed by SPSS packet (one way ANOVA) and the variables were accepted to be defined as significant at 95% of confidence mean interval with p˂0.05. The human semen samples used in this study were voluntarily given and freshly collected by masturbation method after 3days of sexual abstinence from patients attending Reproductive Medicine & Family Planning center of Wuxi Maternal and Child Healthcare Hospital, fertility evaluation and preparation of human semen samples were assessed and treated following the World Health Organization Manual for Analysis and Processing of Human Semen (WHO, 2010) standards to assure the ability of spermatozoa containing in a well-defined fertile semen can reach the fertilization site, it must show a spermatozoal progressive motility ≥40% with sperms mean progressive straight line velocity ( PRᶲVSL) ≥25µm/s [54]. The study was conducted on 30 human semen samples from Chinese males selected during 30days of spring time, only about 1 to 2 semen samples per day were qualified fertile and directly studied for spermatozoa immobilization effect after exposure to coagel without gossypol and to 0.1%, 0.2%, 0.3%, 0.4% and 0.5 % (w/w) of gossypol-containing coagel after different incubation time periods of 0, 5, 10, 15, 20, 25 and 30 minutes all kept at 370 C, all tests were performed within 1hour of semen collection.
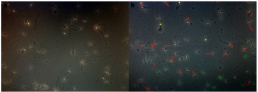
The evaluation of spermicidal activity through immobilization effect was carried out by mixing l00µl of semen with 100µl of each testing coagel, the mixture of semen sample and testing formulation was carefully and completely mixed as quick as possible 2-4 times, a drop of a mixture was suddenly placed on a microscopy glass slide for semen characteristics analysis (Figure 3a) each test was carried out within a 1 to 2 minutes period, if motility of spermatozoa is observed at first time, it is not directly qualified complete till done twice and for samples not showing sperms motility after 2 minutes, a second drop of mixture has been added on slide and observed again for sperm motility, every experiment was repeated 2 times to confirm whether there are or not motile sperms; and the third time in triplicate for recording the average spermatozoa motility percentage correspondingly to incubation period of time. Computer-Assisted/aided Sperms Analyzer-Motility (CASA-Mot.) system (HITMA-IVOS II, Hamilton Thorne Research, USA) was used for triplicate data recording and analysis through which 4 visual fields were taken into considerations by tracking spermatozoa based on their motility levels and labeling them differently into progressive (PR), non-progressive (NP) and immotile (IM) (Figure 3b) and detecting their percentages and other motility related parameters; this is a reliable analysis system recommended for accurate sperm concentration and motility levels analysis [55].
Figure 3:
a) Micrographs of normozoospermic semen (400x)
b) CASA-Motility sperms Analysis: Progressive (PR) in red; Non-Progressive (NP) in green; and Immotile sperms (IM) in yellow
Results and Discussion
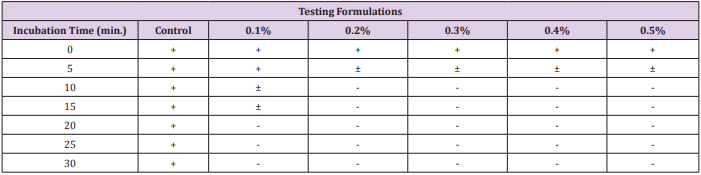
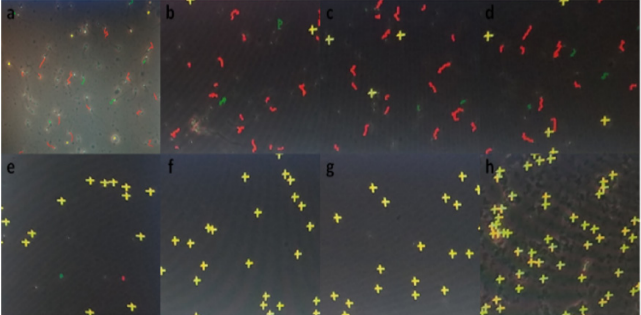
The data recorded to study the changes in number of total progressively motile spermatozoa percentage following an exposure to different concentrations of gossypol-containing coagel over time intervals for deep analysis of decrease in percentage of total motile sperms (Figure 3), illustrative figures were taken and minimum immobilization time was studied (Table 1) by analyzing the required time for a significantly detectable or complete sperms immobilization after exposure to gossypol-containing coagel with different concentrations of gossypol comparably to control. A non-detectable effect or slightly immobilized sperms within 5 minutes was as similarly insignificant with control as well as with all other gossypol-containing formulations (Figure 4a) showing less immobilized sperms before 10min.; as with 0.1% (Figure 4b); 0.2% and 0.3% (Figure 4c); 0.4% and 0.5% (Figure 4d) but highly increasing with time and then becoming significantly visible at high magnification microscopy (400x) through CASA-Motility system at 10min (Figure 4e). With 0.2% likely with 0.3% (Figure 4f) the same as with 0.4% 0.5% (Figure 4g) but; less microspically visible with 0.1% gossypol-containing coagel where sperms or few weak tail shake-like motions were visible (Figure 4e) an importantly complete sperms immobilization was observed between 10-20 and 20-30 minutes; respectively with 0.2%,0.3%, 0.4%, 0.5% and 0.1% (w/w) gossypol-containing coagel, and at the end; the structure of the system in which all the sperms were completely immobilized was identified (Figure 4h).
Table 1: Immobilization time of progressively motile sperms with different concentrations of gossypol.
Note:
a) + : Normal Spermatozoal Progressive Motions.
b) -: No Spermatozoal and Tail Motions.
c) ± : Very weak or tail-shaking motions.
Figure 4: Photomicrographs by CASA, detecting Immobilized human sperms after exposure to test formulations between 0-5min with 0.1%, 0.2%, 0.3%, 0.4% and 0.5% (w/w) gossypol-containing coagel the as between 0-30min with coagel without gossypol
a. After 5-10min with 0.1%
b. With 0.2% and 0.3%
c. With 0.4% and 0.5%
d. And then after 10min. with 0.1%
e. 0.2% the same as 0.3%
f. 0.4% the same as 0.5%
g. And after 30minutes with 0.1%, 0.2%, 0.3%, 0.4% 0.5% of gossypol,
h. the system showing complete immobilization of spermatozoa. The volume ratio of semen /coagel was1:1
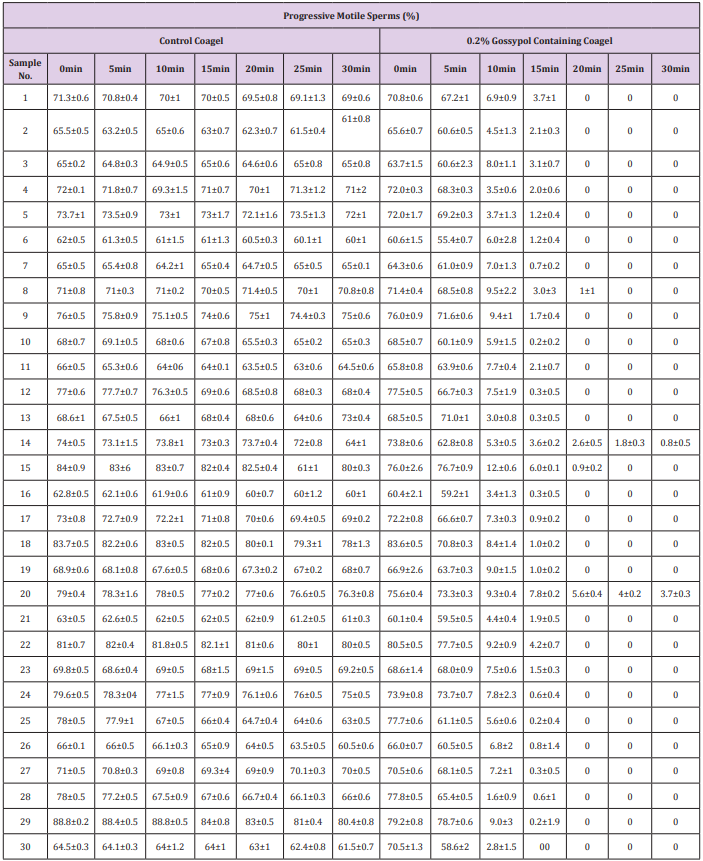
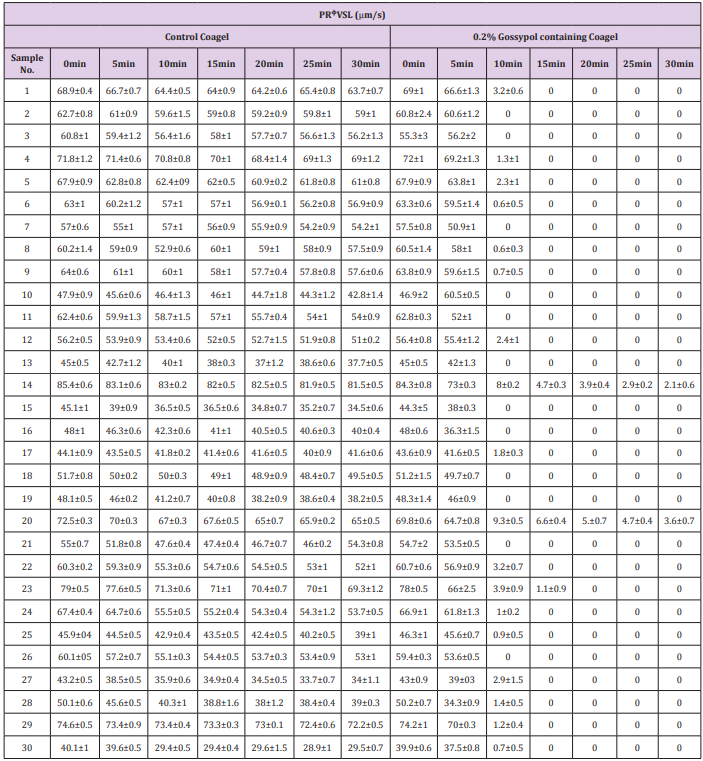
The study of spermatozoa immobilization effects on progressively motile spermatozoal percentage counts (Table 2) and progressive straight line velocity (PRᶲVSL) (Table 3) caused by coagel without and with 0.2% gossypol after 0, 5, 10, 15, 20, 25 and 30 minutes of mixing was concomitantly conducted on 30 semen samples and the data were recorded after a potential analysis by CASA photographic tracking method facilitated by an image analysis hardware and software (Series 3000,ALH,Beats Frequency, Circular Motion; and Research Modules) connected to a video monitor and camera-mounted microscopy; sperms distribution on the slide, sampling variations, and number of video frames tracked were not considered, the mean values of data were recorded from 4 visual fields analyzed by the system. There occurred an indirect but quick decrease in percentage of progressively motile sperms (Table 2) concomitantly with a quite decrease in mean spermatozoal progressive straight line velocity (Table 3) at 10minutes until a complete motility cessation in 28 out of 30 semen samples was observed. The immobilizing effect of different forms of gossypol and its derivatives on human sperms motility has also been reported in many previous studies [9,32,37], but the main mechanism of action of this immobilization is still not clearly known.
Table 2: Effect of 0.2% gossypol-containing coagel on motile sperms percentage.
Note: The progressively motile sperms percentage counts were recorded after 0, 10,15, 20, 25 and 30min of incubation without and with 0.2% gossypol and 0.4% l-Ascorbyl Palmitate coagel without gossypol was used as control. The system considered 4 visual fields during video tracking and these data are expressed as mean ± standard deviation after conducting experience ssin triplicate.
Table 3: Effect of 0.2% gossypol-containing coagel on spermatozoal progressive straight line velocity (PRᶲVSL).
Note: The above values of spermamatozoal PRᶲVSL are expressed as mean ± standard deviation data, there were recorded after 0, 10, 15, 20, 25 and 30min of incubation with 0.2% gossypol-containing coagel and 0.4% l-Ascorbyl Palmitate coagel as control, the data recording was conducted in triplicate and the system considered 4 visual fields during video tracking.
Gossypol may interfere with spermatozoa energy metabolism cycle either by causing uncoupled respiratory chain and oxidative phosphorylation [1,47] or by any other way of reducing sperm cell ATP content [32, 46] and thus lead to decreased motility of spermatozoa of different animals including human in vitro; but if it is orally or parenterally taken leading to decreased sperms counts [30]. The immobilization of human sperms by gossypol incorporated into l-Ascorbyl Palmitate coagel seen in this study, has been shown to be not immediate but rapid after 10 minutes, there has been a 5minutes lag time before a detectable effect is seen, and this may be the fact that the decrease in motility might be quite linked to the perturbation of spermatozoa energy metabolism and may be another unknown mechanism.
The results obtained from our in vitro experiment showed that the first significant sperms immobilization was clearly achieved after 10 minutes with 0.2%, 0.3%, 0.4% and 0.5%,where a great number of progressively motile spermatozoa were seen completely or gradually immobilized and only less than 10% of spermatozoa remained showing a detectable slight head or tail motions after 10 to 20 minutes with 0.1% gossypol-containing coagel, but no effects observed with control; this approved the spermatozoa immobilization effect to be concentration-dependent and a lag time of exposure has appeared before a detectable effect; The two out of 30 different human semen samples treated in this study, only 2 persisted with tail-shaking motions but without forward progression even up to 30minutes of exposure. The results of the present study correlate with results from other studies concerning the immobilizing effect of low doses of gossypol in its appropriate forms where it was reported a small gossypol acetic acid concentration of 10-3M completely abolished spermatozoa motility in vitro once used as cottonseed oil [40]; 2mg of gossypol was able to produce contraceptive effects in vivo rabbits model study [52]; taking into considerations of immobilization time, another study reported a complete immobilization of all spermatozoa by gossypol polyvinyl pyrrolidone co-precipitate at concentrations of 5mg/ml, 40 mg/ml within 3minutes and 20 seconds respectively [9]. More interestingly, human post-coital tests with gossypol-containing vaginal gel application showed a significant spermatozoa number decrease in cervical mucus followed by an immobilization after a period of time [21]. Unfortunately, these data contradict with the results reported by Sukumal et al. [51] where only monkey sperms were completely immobilized within 15 minutes by 50µM of gossypol, but motility of human spermatozoa persisted even at 500µM within 2hours of exposure [43].
Conclusion
In the present case, gossypol incorporated into l-AP showed a strong immobilization effect on human sperms after a reasonable immobilization minimum time of 10 minutes which may be an acceptable spermicidal activity range time necessary to completely abolish sperms motility as a promisingly effective vaginal contraceptive with antimicrobial and antiviral properties. Moreover, the coagel containing 0.2% gossypol may be considered as the most trustworthy and tolerable for future development of vaginal spermicides based on its spermatozoa immobilization activity time and its concentration-related properties as low dose of gossypol-containing formulation. In fact, more in vivo and clinical studies potentiated by nanotechnological development of advanced dosage forms of gossypol-containing coagel to improve its effectiveness and safety are still required for future clinical use.
More BJSTR Articles : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.