The Challenge of Introducing Point of Care Diagnostics in Farm Animal Health Management
Opinion
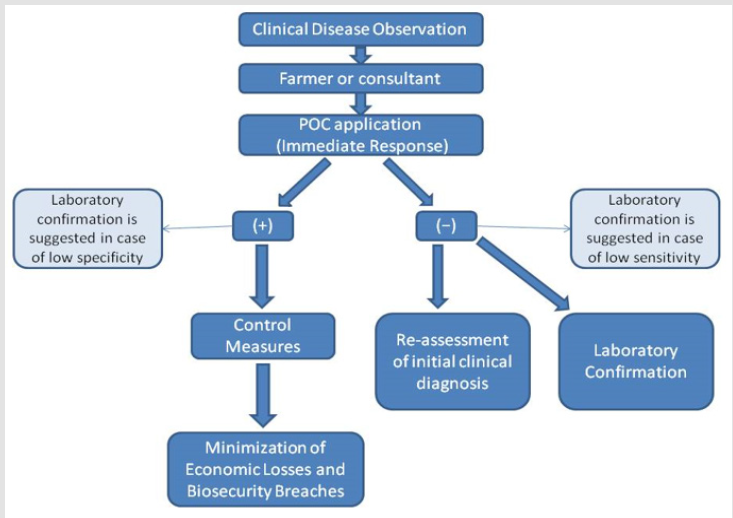
Effective herd health and welfare management is a challenging task for sustainable livestock production. Τhe growing demand for safe and high-quality animal-derived food products, the globalization of trade and the increasing transboundary spread of infectious diseases require early diagnosis and control of biosecurity threats. Rapid, simple, cheap and reliable diagnosis of farm animal diseases directly on-site is an indispensable tool for the development of effective and evidence-based control strategies under the precision livestock farming principles. It is also imperative for the timely diagnosis of endemic or epidemic disease outbreaks, which otherwise can have severe socio-economic consequences (Figure 1). The incorporation of Point of Care (POC) diagnostics in farm animal production, as an integral part of veterinary medical practice, can alleviate to some degree these concerns. POC diagnostics include analytical devices and other rapid tests that provide fast qualitative or quantitative measurements directly on-site [1]. In combination with clinical examination they can be very informative for targeted adjustments on health management schemes.
However, they often require laboratory confirmation due to lower specificity and sensitivity. The World Health Organization (WHO) has recognized the benefits and the drawbacks from using POC applications and for this reason has established a set of criteria describing the ideal POC device [2]. These criteria are indicated by the acronym ASSURED:
Figure 1: Flow diagram of POC testing.
a) Affordable,
b) Sensitive (minimum number of false negatives),
c) Specific (minimum number of false positives),
d) User-friendly (simple to perform),
e) Rapid and Robust,
f) Equipment-free and
g) Deliverable to those who need them.
POC devices and tests are divided in three large categories that include dipstick tests, lateral flow assays (LFA) and microfluidic devices. Dipstick tests provide qualitative detection of the analyte via color change. Lateral flow assays (e.g. pregnancy test) provide qualitative or semi-quantitative detection of the analyte. In these assays, the samples flow via capillary forces through overlapping pads with pre-loaded reagents, for the detection of the analyte [3]. Microfluidic devices are subdivided to Micro Total Analysis Systems (μTAS or Lab-on-a-chip) and microfluidic Paper-based Analytical Devices (μPAD). In μTAS, all the analytical procedures are performed on a single platform. An ideal μPAD should combine the advantages of microfluidic technology with the low cost of paper-based devices.
The first POC applications were developed for human medicine to facilitate the diagnosis of diseases (e.g. diabetes, AIDS) the management of specific conditions (e.g. pregnancy) and the detection of markers (e.g. blood and urine metabolites) [4]. In the case of HIV infections, almost 1,000,000 people die annually, and although the numbers are declining in the developed world, the majority of these deaths occur in developing countries [5]. In this part of the world, the lack of diagnostic laboratories, inadequate logistic capabilities, insufficient infrastructures and trained medical personnel rendered POC testing an attractive alternative for early detection of AIDS. Successful commercialized POC applications in human medicine also include blood glucose-meters and home pregnancy tests. From these observations, one can easily realize that successful development, marketing and commercialization of POC devices and tests require broad societal needs, robust public funds through insurance agencies, and to some degree disposable personal income. These conditions either do not or marginally apply to farm animal health. The application of POC tests in farm animals could provide a powerful tool for herd health management and disease control. In that case, the main advantages from using POC devices under farm conditions are the direct rapid testing and ease of operation [6].
Rapid diagnosis of epidemic diseases is of great importance to prevent severe outbreaks. Currently, the time required for a definite laboratory diagnosis between initial disease outbreak, sample transportation and laboratory confirmation of the etiologic infectious agent can be up to several weeks or months. Thus, the need for the development of portable diagnostic units and/or disposable tests has been recognized. Reliable and simple diagnostic testing directly on-site would enable rapid decision making which is crucial to prevent further spreading of the disease. Additionally, the ease of operation of POC devices allows farmers to perform tests on their own and to take disease control measures instantly. An additional requirement however for POC applications in farm animals is processivity, as commercial farms can have hundreds to even thousands of animals in a confined space. Currently available POC devices seem to suffer from low sensitivity. The most widely used POC devices are Lateral Flow Assays (LFA) such as the human pregnancy test. LFAs show sensitivity as low as 16% when complex biological samples are tested, and the assay is performed by untrained personnel.
On the other hand, their specificity is comparable with laboratory tests. Negative results require further confirmation due to the high number of false negatives [7]. As a result, POC diagnostics with poor sensitivity are not applicable for the diagnosis of diseases such as Foot and Mouth disease (FMD) and African Swine Fever that require drastic measures such as culling (stamping out). Pregnancy tests cost around 10$ each, while rapid HIV antibody screening tests are priced at 8-26$. The fabrication methods used for POC manufacture are often not compatible with mass production. Additionally, POC devices are constructed by costly materials (glass, thermoplastic etc.) [8]. As a result, the end products are relatively costly for animal use. The large number of animals (hundreds or even thousands) required for testing along with the slim margin of profit in animal production (e.g. in poultry production the margin of profit is few euro cents per kilo of meat) make POC testing unaffordable. The development of POC devices with high analytical capability (Lab-on-a-chip, described below) is offered as an alternative. Lab-on-a-chip devices require a larger initial investment but have low operating expenses (consumables). Promising technologies for POC application that could potentially solve some of the inherent problems associated with animal production settings have emerged in the last decade. These technologies could allow affordable testing in a large number of samples with sensitivity and specificity comparable to that of advanced laboratory techniques.
At the forefront are microfluidic devices with microchips that can be now easily manufactured due to advances in microfabrication technologies such as soft lithography and 3D printing. Microfluidic devices allow the manipulation of small sample volumes and can be designed to reliably perform small-scale analytical procedures on a single disposable chip (Lab-on-a-Chip) [9]. Moreover, the recent development of novel biosensors (e.g. cantilever sensors, Geno sensors, electrochemical magnetic microbeads-based biosensors etc.) could also be incorporated in POC devices for veterinary diagnostics that could further improve analytical performance and effectiveness without increasing cost [10]. Technology companies are not always willing of sharing their technological advancements and collaborate with the farming sector due to low profit forecast. Therefore, investments and interdisciplinary research in the field of POC applications in livestock production are limited. Introducing a new POC application requires substantial investments for research, development, validation, commercialization and marketing of the device. Taking into account the capital required to launch a new POC application, the slim margins of profit in animal production and the limited market share, technology companies do not pursue the development of such products. As a result, only few POC applications have been successfully commercialized in animal production.
A better understanding of the farming sector, future market demands and the possible involvement of state funding through subsidies would help initiating research, development and commercialization of critical POC applications [8]. Another major challenge in introducing new POC applications for monitoring animal health is the inability of farmers to exploit new avenues. They are not easily persuaded to invest in modern technologies due to the initial investment cost and require extensive evidence before the decision to adopt a new product. With few exceptions such as the California Mastitis Test (CMT), ketone, urea and pH strip tests, refractometry and ultrasonography for pregnancy diagnosis, POC applications are not yet widely utilized in farm animals. It is therefore imperative that the advantages of new technologies must be better communicated with farmers in order to be understood and fully accepted. This task should be undertaken by the supplier of POC applications and must include extensive training and possible assistance with herd management as part of the service agreement. Another consideration in manufacturing of POC applications for production animals is the limited/non-optimal environmental, energy and labour resources of commercial farm settings.
Exposure to external elements (heat, cold, light, moisture) and inappropriate storage conditions can compromise the device, or the reagents used in the POC application, thus affecting their performance [11]. In addition, farms, especially in remote or underdeveloped areas, may lack energy independence (e.g. electricity, gas-lines). Powering of portable analytical devices is a problem that must be addressed and POC applications should have low energy consumption in order to be functional in such conditions. Powering can be provided with rechargeable batteries, solar panels, biofuel cells and body energy harvesters [12]. Finally, POC devices must provide test results in a simple manner (usually a yes or no answer) in order to be understandable and exploitable by the farmers. Farmers may lack the scientific background to understand complex meanings and to analyse test results. POC devices should operate utilizing a simple interface, whereas, smartphone applications could be used for test result analysis and data extraction.
Conclusion
POC testing is an emerging technology that can be applied successfully in veterinary practice under certain circumstances. POC testing shows advantages, such as rapidity and portability, which can be significant for animal health management. Economic, commercial, environmental and social factors act as crucial regulators in the wider application of POC testing of production animals. The scientific and technological breakthroughs and interdisciplinary research of engineers, biologists and animal experts, are expected to provide new POC applications in the near future. This will safeguard the sustainability and profitability of the farming sector and will aid in the uninterrupted supply of safe and quality animal-derived food products.
Pain Medicine: This is a Time for Neurologists to Take Over the Initiative-https://biomedres01.blogspot.com/2021/03/pain-medicine-this-is-time-for.html
More BJSTR Articles : https://biomedres01.blogspot.com


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.