Direct Access to New Gem-Difluorinated Pyrido[1,2-a] Pyrimidine-2-one Systems
Introduction
The pyrido[1,2-a]pyrimidine core is of great pharmaceutical importance due to its potent and significant biological activities The palmatic acid and stearic acid are the major saturated fatty acids whereas oleic and linoliec acids are unsaturated. The sunflower oil contains more of unsaturated fatty acids, mainly oleic acid and linoleic acid and very less quantity of saturated fatty acid, palmitic acid and stearic acid. Healthy oil should contain more of unsaturated fatty acids compared to saturated fatty acids. The quality of sunflower oil is generally associated with the relative concentration of oleic and linoleic acid. Oil with high oleic acid content has several benefits with regard to human health. High levels of saturated fat (Palmitic and stearic acid) consumption are correlated with increased risk of coronary heart disease. Oils with high oleic content are resistant to heat oxidation, longer shelf life and low cholesterol effect. However, Roberston reported that linoleic acid content varied inversely with the oleic acid content.
High proportion of the essential fatty acid (18:2) is considered to reduce blood cholesterol and hence sunflower has a special significance. High linoleic sunflower with 70% linoleic acid is also available in studied germplasm. It has greater oxidative stability and is useful as frying oil in the preparation of snack foods. Furthermore, sunflower oil contains fat soluble vitamins A, B, E and K, well for heart proteins [1]. The objective of this study was to make a review of the genetic variability of oil quality components in sunflower and inheritance pattern in cross combination using own results and those of other authors.
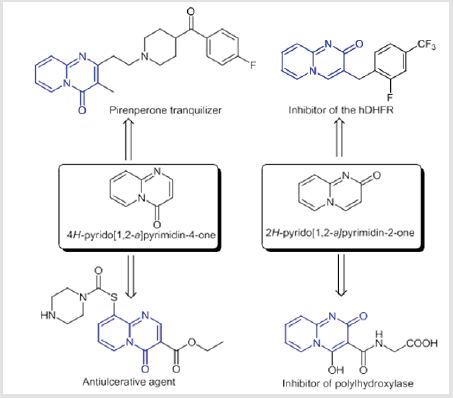
In view of the importance of the 2H-pyrido[1,2-a]pyrimidin- 2-one scaffold, our laboratory team studied the extension of this heterocyclic family. We discovered a new series of synthesized molecules exhibiting in vitro activity against the host cell invasion by E. tenella parasites. This investigation revealed a “chief” compound with a “pyrido[1,2-a]pyrimidin-2-one ”, with an IC50 value of 15 μM.[11] Furthermore, it is well established that the incorporation of a fluorine atom into organic compounds can alter or modify their physiochemical properties and enhance their metabolic stability. The unique properties of fluorine have an exceptional impact on the electronic, lipophilic, and steric parameters, and acidity or basicity of organofluorinated molecules.[12] Much attention has been drawn in recent years to the difluoromethylene group (CF2) due to its special physical and chemical properties.[13]
Methods
General Methods
Most reagents were obtained from commercial sources and used as received. Thin-Layer Chromatography (TLC) was performed on Merck 60F254 plates. Column Chromatography was carried out with Merck silica gel 60 (0.040-0.063 mm, 230-400 mesh). All 1H NMR, 13C NMR and 19F NMR spectra were recorded on a 300 MHz Bruker Avance FT- NMR spectrometer (300 MHz, 75 or 282 MHz, respectively). All chemical shifts are given as δ value (ppm) with reference to tetramethylsilane (TMS) as an internal standard. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. The coupling constants J are reported in Hertz (Hz). Electrospray ionization High-Resolution Mass Spectrometry Experiments (HRMS) were performed on a hybrid Tandem Quadrupole/Time-Of Flight (Q-TOF) instrument, equipped with a pneumatically assisted electrospray (Z-spray) ion source (Micromass, Manchester, U.K.) operated in positive mode.
Results and Discussion
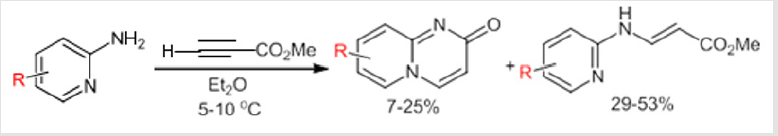
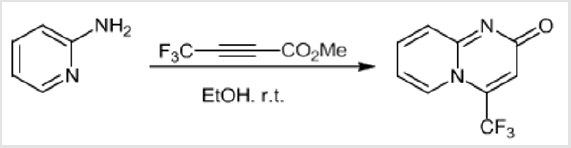
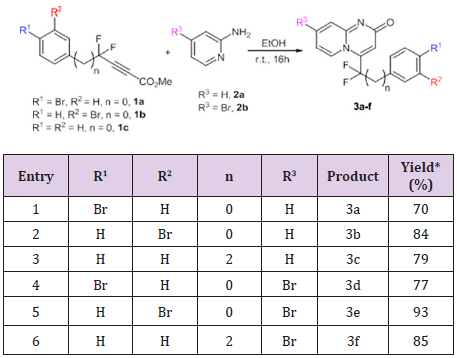
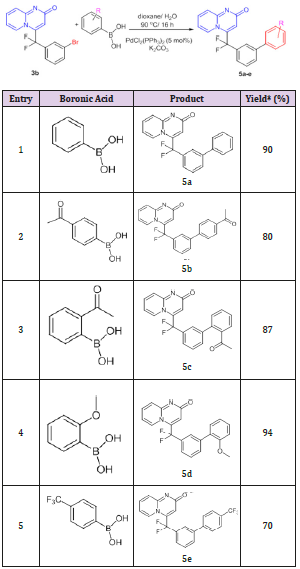
Due to the valuable role of 2H-pyrido[1,2-a]pyrimidin-2-one derivatives as well as the important pharmacological role of the CF2 moiety, we planed to look for an access to new gem-difluorinated-2H-pyrido[1,2-a]pyrimidin-2-one derivatives. Our investigation started by exploring the cycloaddition reaction between two different 2-aminopyridines and gem-difluorinated alkynes. The corresponding gem-difluorinated alkynes were prepared in the laboratory following a known synthetic strategy.[14] The cycloaddition reaction was done in ethanol at room temperature. Following the same mechanism as that described by Harriman’s group.[10] The cycloaddition reactions between three different gem-difluorinated alkynes 1a-c and 2-aminopyridines 2a-b were successfully conducted, leading to the formation of pyrido[1,2-a] pyrimidin-2-ones 3a-f with good to excellent yields (70-93%) (Table 1). Moreover, the reactions proceeded very cleanly, and the desired products were obtained as a solid in pure form after filtration. As product 3b bears a reactive carbon-bromide bond connected to the phenyl group, it seemed important to test the reactivity of this bond in order to achieve further transformations. Therefore, a palladium catalyzed Heck coupling reaction was carried out on pyrido[1,2-a] pyrimidin-2-one 3b with methyl acrylate.
Table 1: Cycloaddition reaction between gem-difluorinated alkynes and 2-aminopyridines.
*=Isolated yield
The best conditions for performing this coupling reaction were found to be a catalytic amount of palladium (II) acetate [Pd(OAc)2, 5 mol%], triphenyl phosphine [PPh3, 10 mol%] and triethylamine as base and heating in a sealed tube at 120°C. The reaction gave the desired product 4 (E/Z = 94/6) that was easily purified by recrystallization and was isolated with a 60% of overall yield. A typical Suzuki-Miyaura cross coupling reaction[15] was then tested on pyrido[1,2-a]pyrimidin-2-one 3b using a variety of phenyl boronic acids in order to achieve further transformation. The reactions were performed in a mixture of dioxane/water (3/1) with Palladium (II)bis(triphenylphosphine) dichloride [PdCl2(PPh3)2] (5 mol%) as catalyst and Potassium carbonate (K2CO3) as base. The desired products 5a-e were isolated in a yield ranging between 70-94%. The results are summarized in Table 2. Despite of their ability to facilitate the oxidative addition step in Suzuki-Miyaura cross coupling reaction, the presence of EWG such as a CF3 group (entry 5, Table 2) in phenyl boronic acids has the disadvantage to induce a partial protodeboronation in presence of aqueous base which globally decreases the yield of the cross-coupling reaction.[16-18].
Table 2: Suzuki coupling reaction of 4-[(3-bromophenyl) difluoromethyl]-2H-pyrido[1,2-a]pyrimidin-2-one 3b.
*=Isolated yield
Experimental Part
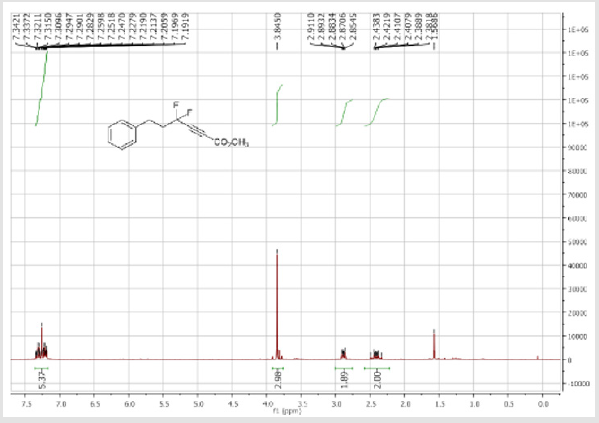
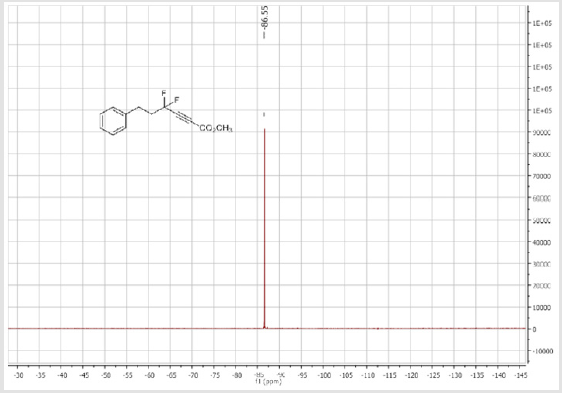
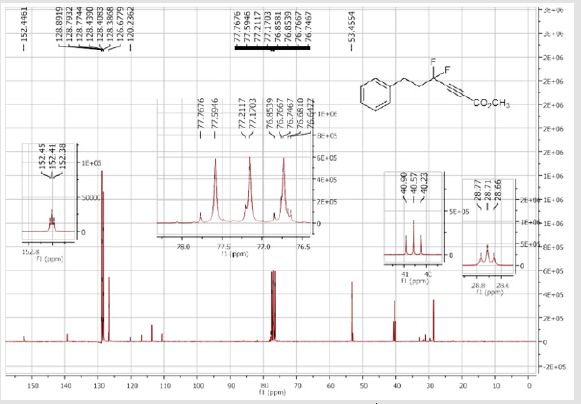
Methyl 4,4-difluoro-6-phenylhex-2-ynoate (1c): Colorless oil; C13H12F2O2; yield = 70%; 1H NMR (300 MHz, CDCl3): δ (ppm) = 7.34-7.19 (m, 5H), 3.84 (s, 3H), 2.81-2.83 (m, 2H), 2.46- 2.29 (m, 2H). 13C NMR (75 MHz, CDCl3): δ (ppm) = 152.3 (t, CO, JCF = 2.4 Hz), 139.2, 128.8 (CH), 128.7 (2CH), 128.4 (2CH), 113.7 (t, CF2, JCF = 234.7 Hz), 77.2 (t, JCF = 40.7 Hz), 76.8 (t, JCF = 6.7 Hz), 53.4 (CH3), 40.5 (t, CH2, JCF = 25.1 Hz), 28.7 (t, CH2, JCF = 4.0 Hz). 19F NMR (282 MHz, CDCl3): δ (ppm) = - 86.53. HRMS(ESI): m/z [M+H] +calcd for C13H12F2O2: 239.08781; found: 239.08736.
General procedure for cycloaddition reaction between fluorinated alkynes 1a and 2-aminopyridine derivatives: 2-Aminopyridine (12 mg, 0.13 mmol) in 2 ml of ethanol was introduced in a small round bottom flask. The gem difluorinated alkyne 1a (41 mg, 0.14 mole, 1.1 equiv.) was added drop by drop. Once the reaction was completed, the solvent was evaporated under vacuum. The product was rinsed with ether and then dried under vacuum yielding the pure product 3a without any additional treatment.
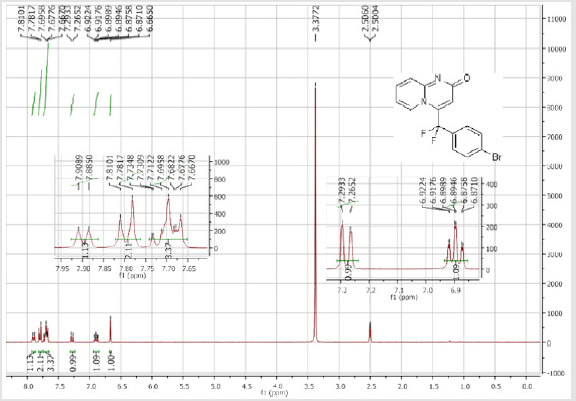
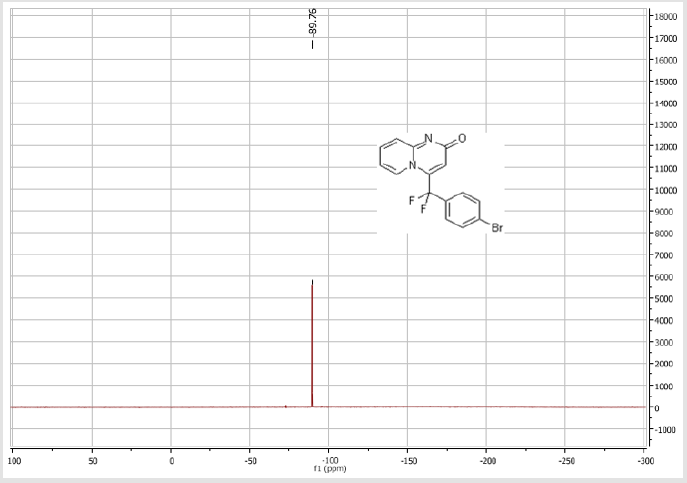
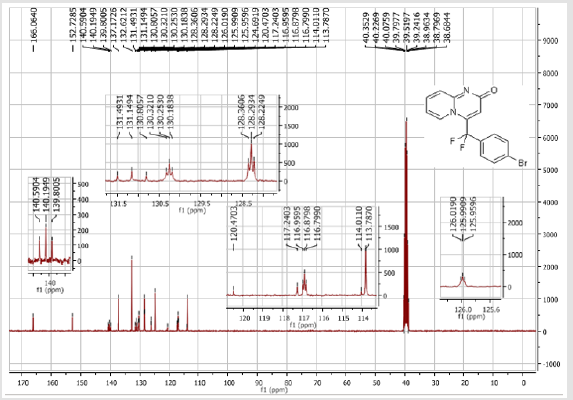
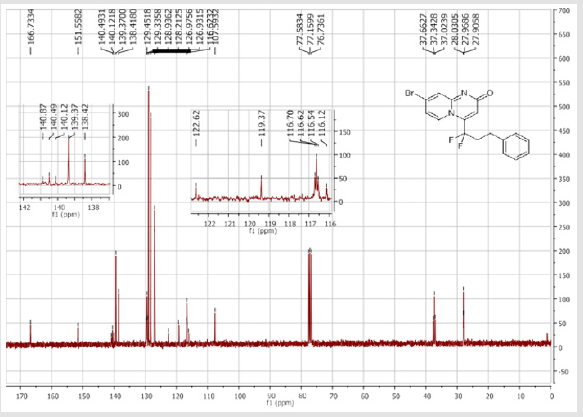
4-[(4-Bromophenyl)difluoromethyl]-2H-pyrido[1,2-a] pyrimidin-2-one (3a): Yellow soli; C15H9BrF2N2O; Yield = 70%; m.p = 219-221oC. 1H NMR (300 MHz, DMSO-d6): δ (ppm) = 7.90 (d, 1H, J = 7.2 Hz), 7.79 (d, 2H, J = 8.5 Hz), 7.73-7.67 (m, 3H), 7.28 (dd, 1H, J = 9.9/1.2 Hz), 6.90 (dt, 1H, J = 1.2/7.2 Hz), 6.66 (s, 1H). 13C NMR (75 MHz, DMSO-d6): δ (ppm) = 166.1 (CO), 152.7, 140.2 (t, JCF = 29.7 Hz), 137.2 (CH), 132.6 (2CH), 131.1 (t, JCF = 25.8 Hz), 130.2 (t, CH, JCF = 5.2 Hz), 128.3 (t, 2CH, JCF = 5.1 Hz), 126.0 (t, JCF = 2.1 Hz), 124.7 (CH), 117.2 (t, CF2, JCF = 241.9 Hz), 116.9 (t, CH, JCF = 6.0 Hz), 113.8 (CH). 19F NMR (282 MHz, DMSO-d6): δ (ppm) = - 89.76. HRMS(ESI): m/z [M+H] +calcd for C15H10BrF2N2O2: 350.99291; found: 350.99237.
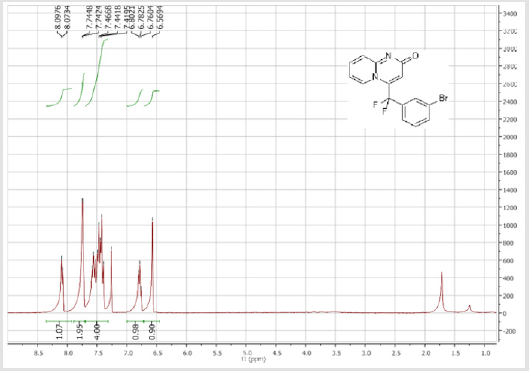
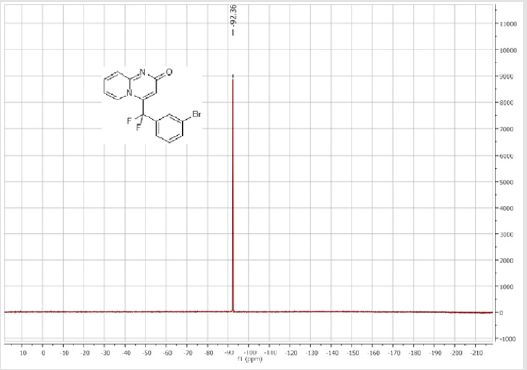
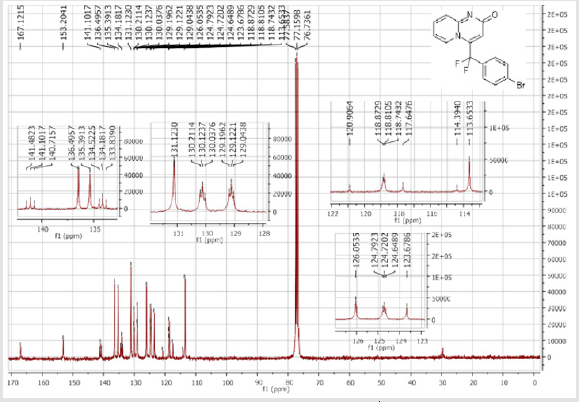
4-[(3-Bromophenyl)difluoromethyl]-2H-pyrido[1,2-a] pyrimidin-2-one (3b): Yellow solid; C15H9BrF2N2O; Yield = 84%; m.p = 219-221oC. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.05 (d, 1H, J = 7.3 Hz), 7.74 (m, 2H), 7.55-7.39 (m, 4H), 6.78 (dt, 1H, J = 1.3/7.3 Hz), 6.57 (s, 1H). 13C NMR (75 MHz, CDCl3): δ (ppm) = 167.1 (CO), 153.2, 141.1 (t, JCF = 28.5 Hz), 136.5 (CH), 136.4 (CH), 134.2 (t, JCF = 25.7 Hz), 131.1 (CH), 130.1 (t, CH, JCF = 6.5 Hz), 129.1 (t, CH, JCF = 5.9 Hz), 126.1 (CH), 124.7 (t, CH, JCF = 5.4 Hz), 123.7 (CH), 118.8 (t, CH, JCF = 4.7 Hz), 117.6 (t, CF2, JCF = 244.4 Hz), 133.6. 19F NMR (282 MHz, CDCl3): δ (ppm) = - 89.76. HRMS(ESI): m/z [M+H]+calcd for C15H9BrF2N2O: 350.99391 ; found: 350.99507.
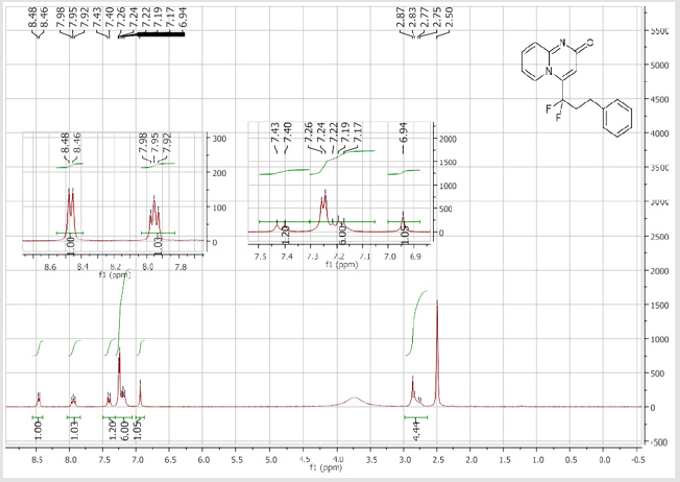
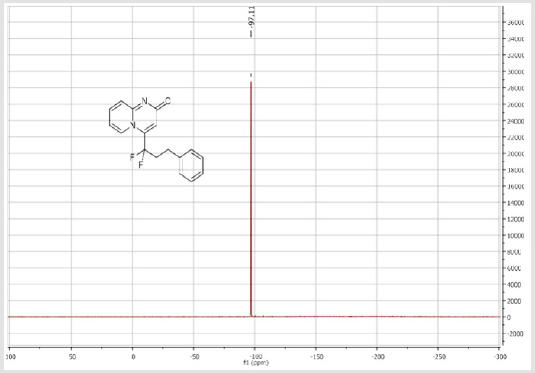
4-(1,1-Difluoro-3-phenylpropyl)-2H-pyrido[1,2-a] pyrimidin-2-one (3c): Yellow solid; C17H14F2N2O; Yield = 79%; m.p = 120-122oC. 1H NMR (300 MHz, DMSO-d6): δ (ppm) = 8.47 (t, 1H, J = 7.1 Hz), 7.95 (t, 1H, J = 7.8 Hz), 7.41 (d, 2H, J = 9 Hz), 7.26-7.17 (m, 5H), 6.94 (s, 1H), 2.88-2.75 (m, 4H). 13CNMR (75 MHz, DMSO-d6): δ (ppm) = 163.4 (CO), 151.6, 141.2 (t, JCF = 28.4 Hz),
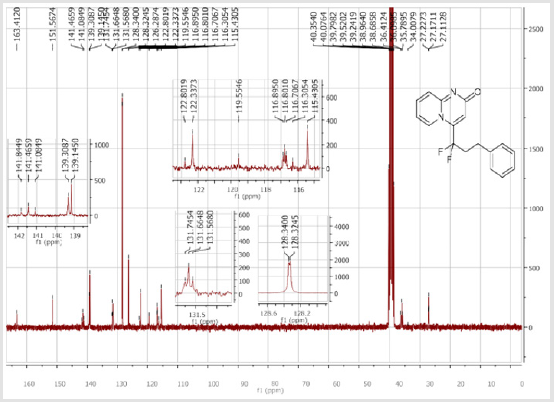
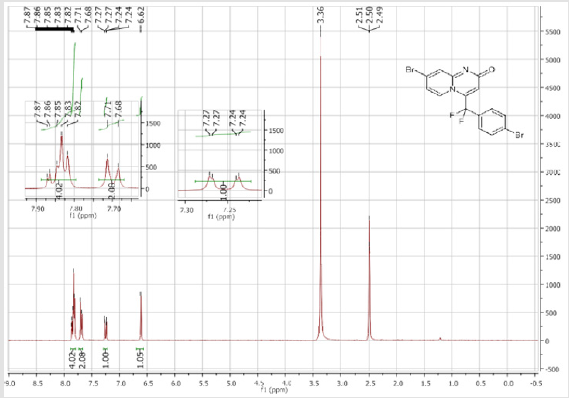
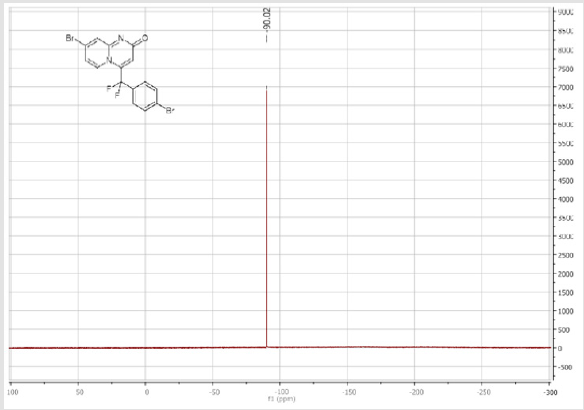
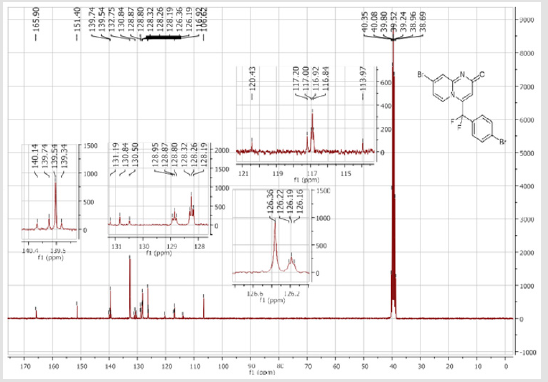
139.3 (CH), 139.1 (CH), 131.7 (t, JCF = 6.6 Hz), 128.4 (2CH), 128.3 (2CH), 126.3 (CH), 122.3 (CH), 119.6 (t, CF2, JCF = 245.4 Hz), 116.8 (t, CH, JCF = 7.0 Hz), 115.4 (CH), 36.10 (t, CH2, JCF = 23.2 Hz), 27.2 (t, CH2, JCF = 4.6 Hz). 19F NMR (282 MHz, DMSO-d6): δ (ppm) = - 95.70. HRMS (ESI): m/z [M+H] +calcd for C17H15F2N2O2: 301.11470; found: 301.11340.8-Bromo-4-[(4-bromophenyl) difluoromethyl]- 2H-pyrido[1,2-a]pyrimidin-2-one (3d): Yellow solid; C15H8Br2F2N2O; Yield = 77%; m.p. =200-202oC. 1H NMR (300 MHz, CDCl3): δ (ppm) = 7.87-7.82 (m, 4H), 7.70 (d, 2H, J = 8.6 Hz), 7.25 (dd, J = 1.0/9.3 Hz, 1H), 6.62 (s, 1H). 13C NMR (75 MHz, CDCl3sub>): δ (ppm)= 165.9 (CO), 151.4, 139.7 (t, JCF = 29.7 Hz), 139.5 (CH), 132.7 (2CH), 130.8 (t, JCF = 25.8 Hz), 128.9 (t, CH, JCF = 5.7 Hz), 128.2 (t, 2CH, JCF = 5.2 Hz), 126.4, 126.2 (t, CH, JCF = 2.3Hz), 117.2 (t, CF2sub>, JCF = 241.7 Hz), 111.9 (t, CH, JCF = 5.8 Hz), 106.6 (CH). 19F NMR (282 MHz, CDCl3sub>): δ (ppm) = -90.02. HRMS(ESI): m/z [M+H] +calcd for C15H9Br2F2N2O: 428.90442; found: 428.90269.
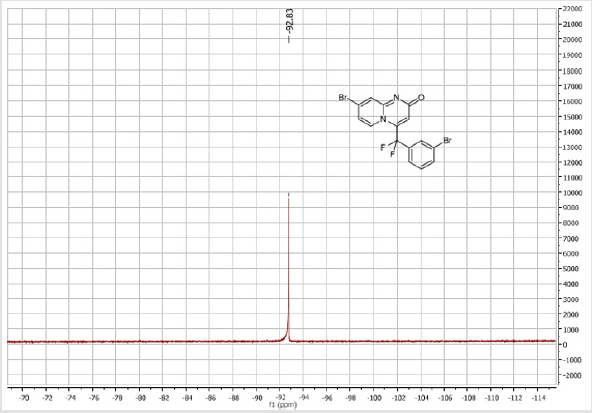
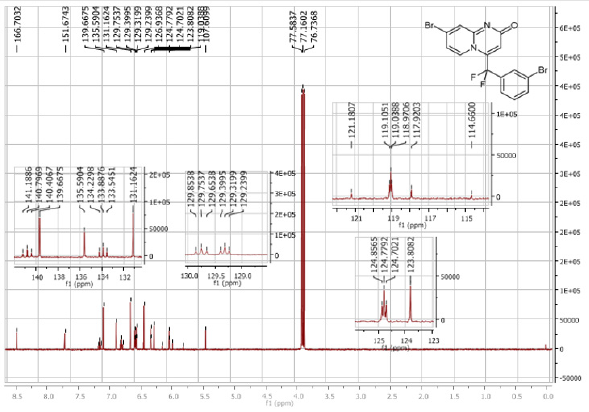
7-Bromo-4-[(3-bromophenyl)difluoromethyl]- 2H-pyrido[1,2-a]pyrimidin-2-one (3e): Yellow solid; C15H8Br2F2N2O; Yield = 93%; m.p. = 200-202oC. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.27 (s, 1H), 7.75 (d, J = 7.6 Hz, 1H), 7.73 (t, J= 7.6 Hz, 1H), 7.59 (dd, J = 1.6/9.6 Hz, 1H), 7.51-7.41 (m, 2H), 7.29 (m, 1H), 6.46(s, 1H). 13C NMR (75 MHz, CDCl3): δ (ppm)= 166.7 (CO), 151.7, 140.8 (t, JCF = 29.4 Hz), 139.7, 135.6, 133.9 (t, JCF= 25.7 Hz), 131.2, 126.7 (t, JCF = 7.5 Hz), 129.3 (t, JCF = 6.0 Hz), 126.9, 124.8 (t, JCF = 5.8 Hz), 123.8, 119.0 (t, JCF = 5.0 Hz), 117.9 (t, CF2, JCF = 244.5 Hz), 107.6. 19F NMR (282 MHz, CDCl3): δ (ppm) = - 92.83. HRMS(ESI): m/z [M+H] +calcd for C15H9Br2F2N2O: 428.90442; found: 428.90632.
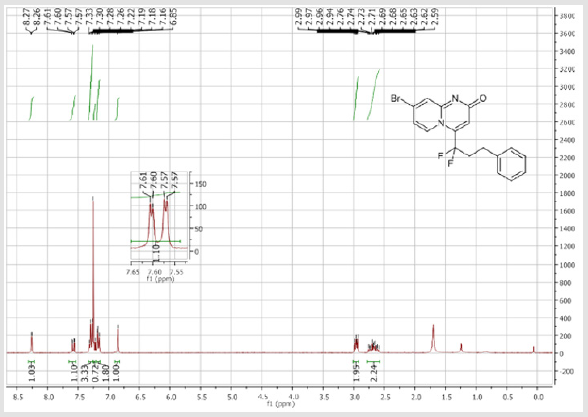
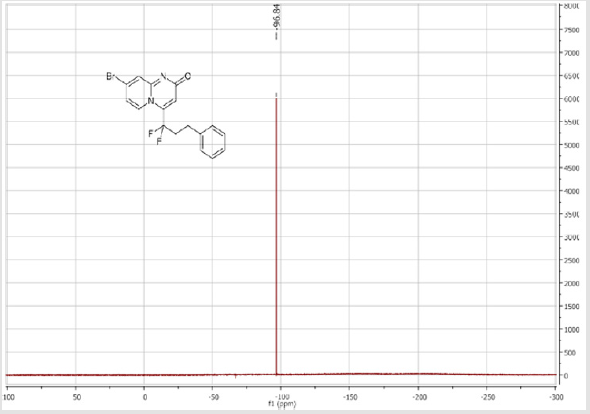
8-Bromo-4-(1,1-difluoro-3-phenylpropyl)-2H-pyrido[ 1,2-a]pyrimidin-2-one (3f): Yellow solid; C17H13BrF2N2O; Yield = 85%; m.p. =146-148oC. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.26 (d, 1H, J = 1.7 Hz), 7.59 (dd, 1H, J = 1.8/9.6 Hz,), 7.33-7.16 (m, 6H), 6.85 (s, 1H), 2,97 (m, 2H), 2.69 (m, 2H). 13C NMR (75 MHz, CDCl3sub>): δ (ppm) = 166.7 (CO), 151.6, 140.5 (t, JCF = 28.5 Hz), 139.4 (CH), 138.4, 129.4 (t, CH, JCF = 8.7 Hz), 128.2 (2CH), 128.2 (2CH), 126.8 (CH), 126.9 (CH), 119.4 (t, CF2, JCF = 243.5 Hz), 116.6 (t, JCF = 6.1 Hz), 107.6(CH), 37.3 (t, CH2, JCF = 24.0 Hz), 28.0 (t, CH2, JCF = 4.6 Hz). 19F NMR (282 MHz, CDCl3): δ (ppm) = -95.84. HRMS(ESI): m/z [M+H] +calcd for C17H14BrF2N2O: 379.02340; found: 379.02340.
Procedure of Heck Reaction for Preparation of Compound 4: Compound 3b (30 mg, 0.08 mmol), triphenyl phosphine PPh3 (2.2 mg, 10 mol%), and Palladium (II) acetate [Pd(OAc)2] (1 mg, 5 mol%) were introduced in a sealed tube with an excess of methyl acrylate. Then trimethylamine [Et3N] (0.04 ml) was added slowly. The reaction mixture was set at 120oC under Argon. After the reaction was completed, extraction using ethyl acetate and water was done. The reaction mixture was dried over Magnesium sulfate and the solvent was evaporated under vacuum. The pure product was isolate using silica gel column chromatography in 60% yield (18.2 mg, yellow solid). m.p = 175-177oC.
Methyl-3-[3-(difluoro(2-oxo-2H-pyrido[1,2-a] pyrimidin-4-yl]methyl)phenyl)acrylate (4-E): C19H14F2N2O3; 1HNMR (300 MHz, CDCl3): δ (ppm) = 8.10 (d, J = 7.0 Hz, 1H), 7.73 (d, J = 6.8 Hz, 1H), 7.71 (s, 1H), 7.68 (d, 1Halkene, J = 16.0 Hz), 7.56-7.55 (m, 3H), 7.40 (d, J = 9.1 Hz, 1H), 6.72 (dt, J = 1.4/7.1 Hz, 1H), 6.52 (s, 1H), 6.43 (d, J = 16.0 Hz, 1H), 3.75 (s, 3H). 19F NMR (282 MHz, CDCl3): δ (ppm) = -92.47. HRMS(ESI): m/z [M+H]+calcd for C19H15F2N2O3: 357.10453; found: 357.10416.
General Procedure of Suzuki Cross Coupling Reaction on Compound 3b: Compound 3b (30 mg, 0.085 mmole), phenyl-boronic acid (42 mg, 0.34 mmole, 4 equiv.), Palladium(II) bis(triphenylphosphine) dichloride PdCl2sub>(PPh3)2> (0.5 mol%), Potassium carbonate [K2CO3] (23.5 mg, 0.17 mmole, 2 equiv.), and a mixture of dioxane/H2O (3/0.6 ml) were introduced in a sealed tube. The reaction mixture was heated to 90°C. When the reaction was finished, Sodium chloride [NaCl] was added. Extraction was done with ethyl acetate and water. The organic phase was dried over Magnesium sulfate, filtered followed by the evaporation of the solvent. The desired product 5a was isolated in 90% yield (26.5 mg, white solid) using silica gel column chromatography, eluent EA/ PE = 9/1.
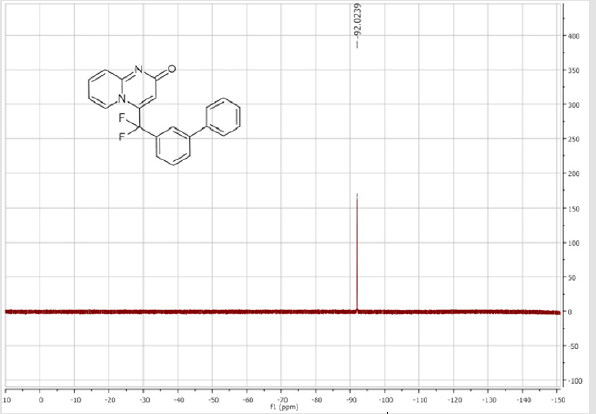
4-([1,1’-Biphenyl]-3-yl] difluoromethyl-2H-pyrido[ 1,2-a]pyrimidin-2-one (5a): White solid; C21H14F2N2O; m.p.= 199-201°C. 1H NMR (300 MHz, CDCl3sub>): δ (ppm) = 8.18 (d, 1H, J = 7.0 Hz), 7.82 (d, 1H, J = 7.0 Hz), 7.77 (s, 1H), 7.63-7.40 (m, 8H), 7.27 (t, 1H, J = 7.0 Hz), 6.80 (t, 1H, J = 7.0 Hz), 6.72 (s, 1H). 19F NMR (282 MHz, CDCl3): δ (ppm) = - 92.02. 13C NMR (75 MHz, CDCl3): δ (ppm) = 167.1 (CO), 153.1, 142.8,141.5 (t, JCF = 30.4 Hz), 139.3, 136.1 (CH), 130.8 (t, CH, JCF = 9.8 Hz), 130.8 (CH), 130.3 (t, CH, JCF = 6.9 Hz),129.9 (CH), 129.1 (2CH), 128.3 (CH), 127.2 (2CH), 129.9, 124.6 (t, CH, JCF = 5.8 Hz), 124.5 (t, CH, JCF = 5.2 Hz),118.7 (t, CH, JCF = 5.3 Hz), 118.4 (t, CF2, JCF = 244.3 Hz), 113.2 (CH). HRMS(ESI): m/z [M+H]+calcd for C21H15F2N2O: 349.1042 ; found: 349.11409.
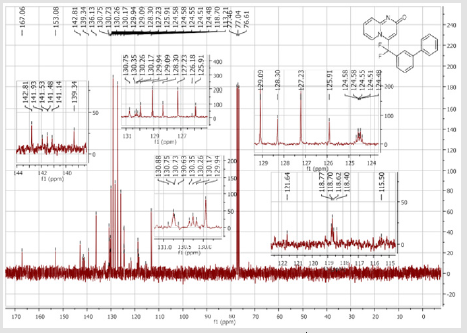
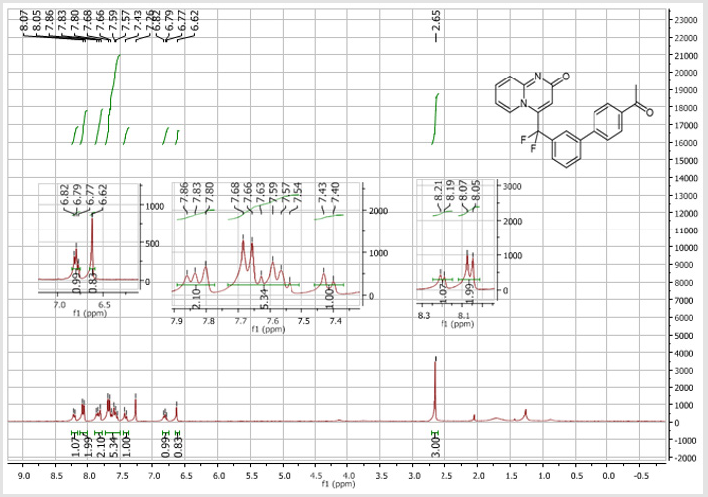
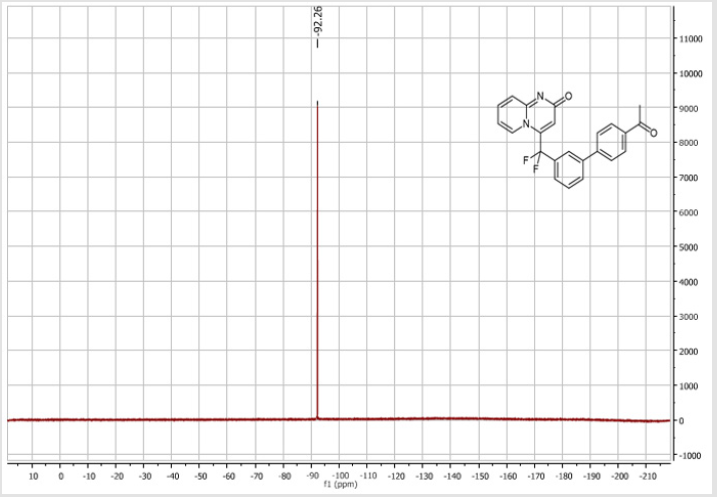
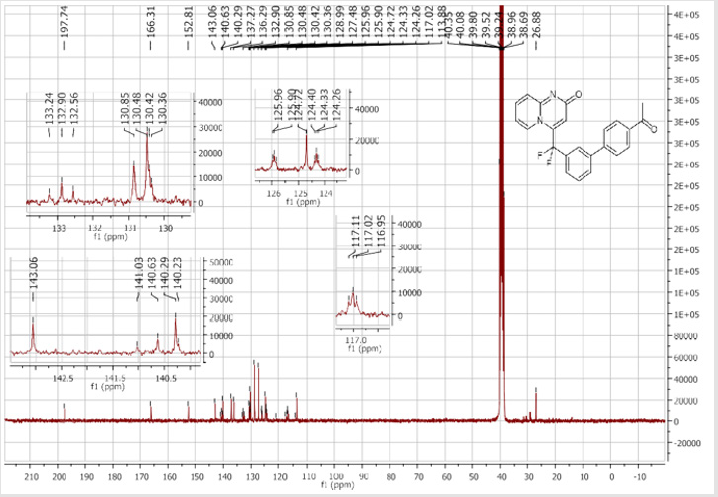
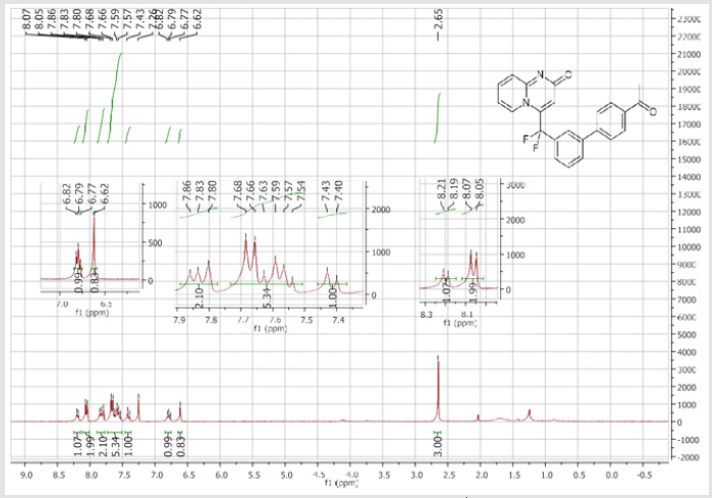
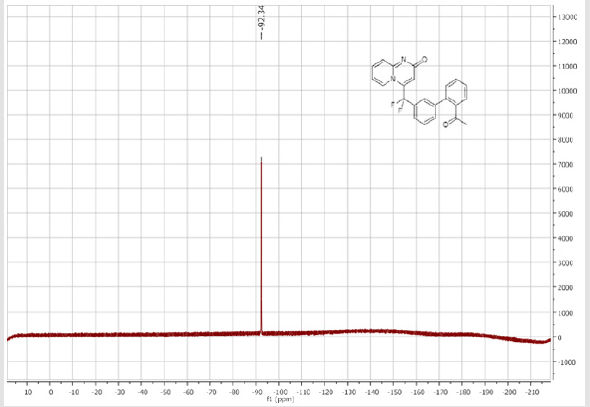
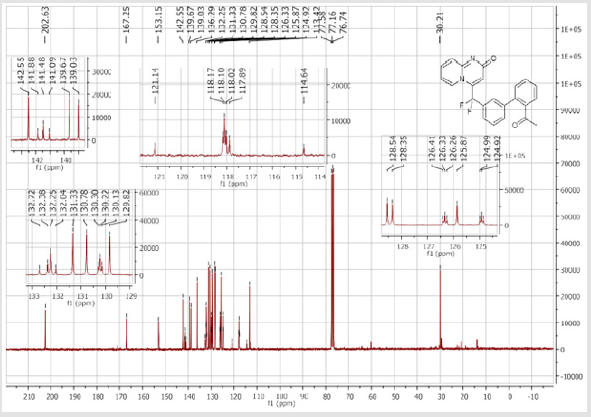
4-[(4’-Acetyl-[1,1’-biphenyl]-3-yl)difluoromethyl]- 2H-pyrido[1,2-a]pyrimidin-2-one (5b): Yellow solid; C23H16F2N2O2; Yield = 80%; m.p. = 215-217°C. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.20 (d, J = 7.1 Hz, 1H), 8.06 (t, J = 8.3 Hz, 2H), 7.84 (d, J= 7.5 Hz, 1H), 7.80 (bs, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.62- 7.55 (m, 3H), 7.41 (d, J = 9.0 Hz, 1H), 6.79 (dt, J = 6.9/1.0 Hz, 1H), 6.62 (s, 1H), 2.65 (s,3H). 13C NMR (75 MHz, DMSO- d6): δ (ppm) = 197.8 (COCH3), 166.3 (CO), 152.8, 143.1, 140.4 (t, JCFF2N2O2;> = 25.8 Hz), 140.3, 137.3, 136.3 (CH), 132.9 (t, JCF = 25.3 Hz), 130.9 (CH), 130.5 (CH), 130.4 (t, CH, JCF = 5.7 Hz), 129.0 (2CH), 127.5 (2CH), 125.9 (t, CH, JCF = 5.2 Hz), 124.7 (CH), 124.3 (t, CH, JCF = 5.2 Hz), 177.5 (t, CF2, JCF = 241.3 Hz), 117.0 (t, CH, JCF = 5.7 Hz), 113.9 (CH), 26.9 (CH3). 19F NMR (282 MHz, DMSO-d6): δ (ppm) = - 92.26. HRMS(ESI): m/z [M+H]+calcd for C23H17F2N2O2: 391.12526; found: 391.12649.
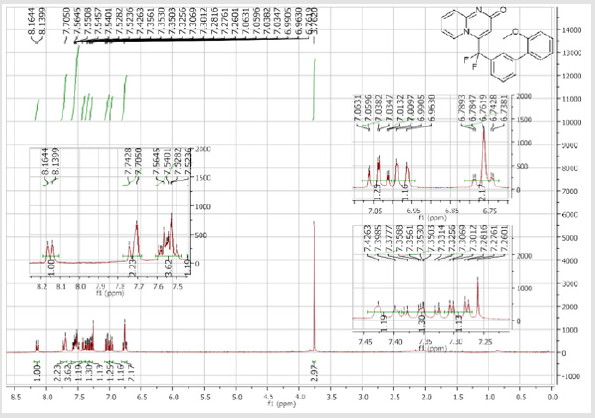
4-[(2’-Acetyl-[1,1’-biphenyl]-3-yl)difluoromethyl]- 2H-pyrido[1,2-a]pyrimidin-2-one (5c): Yellow solid; C23H16F2N2O2 Yield = 87%; m.p. = 215-217°C. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.06 (d, J = 7.2 Hz, 1H), 7.64 (dd, J = 1.2/ 7.5 Hz, 1H), 7.58-7.48 (m, 7H), 7.41-7.34 (m, 2H), 6.79 (dt, J = 1.5/6.9 Hz, 1H), 6.74 (s, 1H), 2.20 (s, 3H). 13C NMR (75 MHz, CDCl3): δ (ppm) = 202.6 (COCH3), 167.2 (CO), 153.1, 142.5, 141.5 (t, JCF =29.8 Hz), 139.7, 139.0, 136.3 (CH), 132.4 (t, JCF= 25.3 Hz), 132.2 (CH), 131.3 (CH), 130.8 (CH), 130.2 (t, CH, JCF= 6.0 Hz), 129.8 (CH), 128.5 (CH), 128.4 (CH), 126.3 (t, CH, JCF = 5.6 Hz), 125.9 (CH), 124.9 (t, CH, JCF = 5.4 Hz), 118.1 (t, CH, JCF = 5.5 Hz), 117.9 (t, CF2, JCF = 243.6 Hz), 113.4 (CH), 30.2 (CH3). 19F NMR (282 MHz, CDCl3): δ (ppm) = -92.33. HRMS (ESI): m/z [M+H] + calcd for C23H17F2N2O2: 378.12426; found: 378.12653.
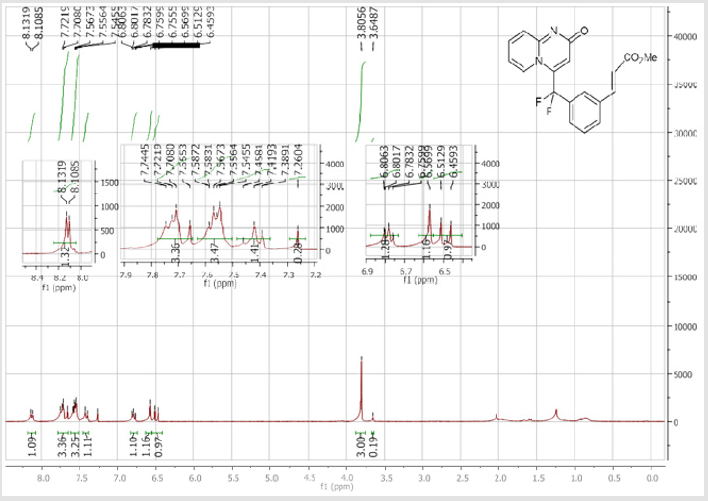
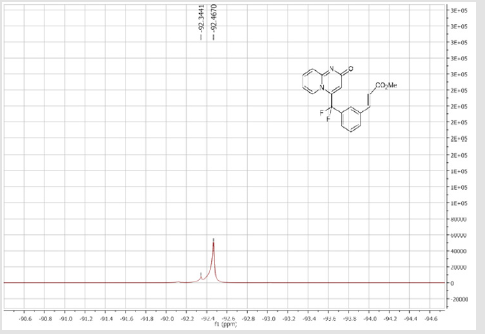
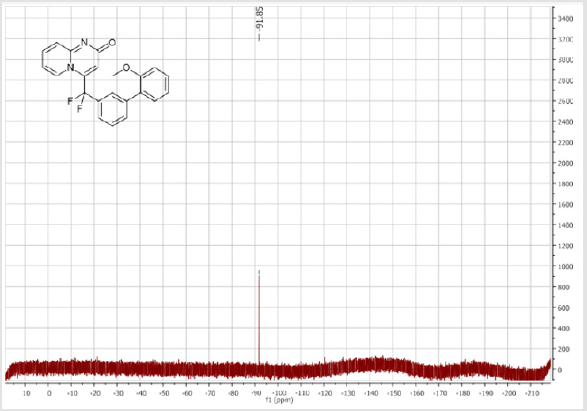
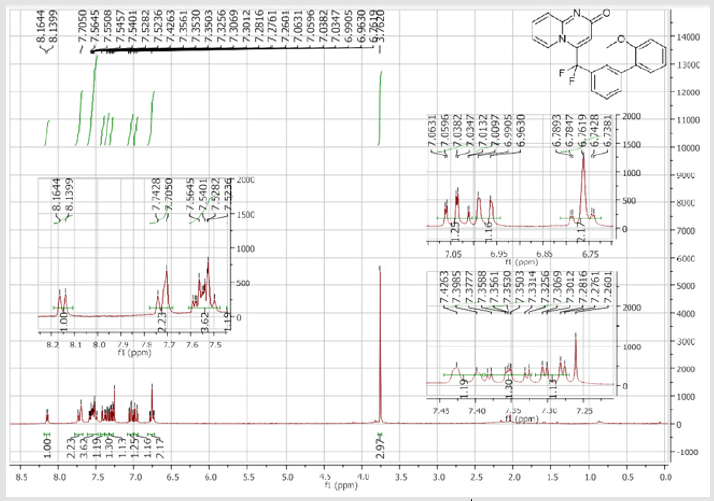
4-(Difluoro(2’-methoxy-[1,1’-biphenyl]-3-yl) methyl)-2H-pyrido[1,2-a]pyrimidin-2-one: Yellow solid; C22H16F2N2O2; Yield = 94%; m.p. = 201-203°C. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.05 (d, J = 7.2 Hz, 1H), 7.63 (d, J = 8.8 Hz, 1H), 7.62 (s, 1H), 7.49-7.40 (m, 3H), 7.32-7.18 (m, 3H), 6.96-6.87 (m, 2H), 6.67 (s, 1H), 6.66 (m, 1H), 3.67 (s, 3H). 13C NMR (75 MHz, CDCl3): δ (ppm) = 171.2 (CO), 167.4, 156.3, 153.1, 141.7 (t, JCF= 29.7 Hz), 139.9, 136.2 (CH), 133.1 (CH), 131.7 (t, JCF = 25.1 Hz), 130.6 (CH), 130.4 (t, CH, JCF = 6.4 Hz), 129.7(CH), 129.2 (CH), 128.6 (CH), 127.2 (t, CH, JCF = 5.6 Hz), 125.8 (CH), 124.1 (t, CH, JCF = 5.5 Hz), 121.1 (CH), 118.5 (t, CH, JCF = 5.5 Hz), 118.4 (t, CF
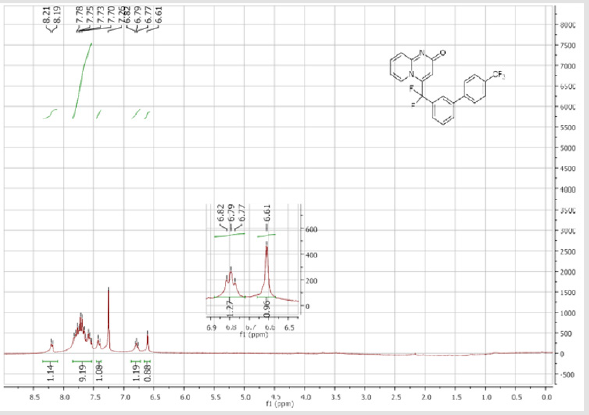
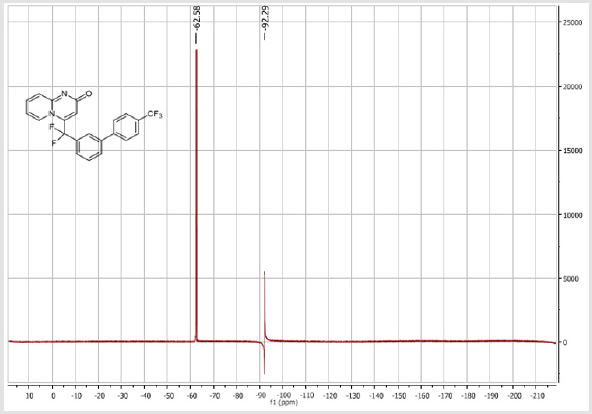
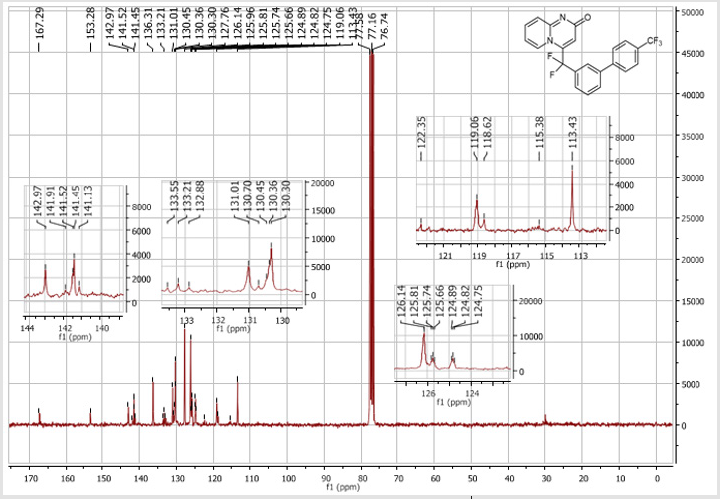
4-(Difluoro(4’-(trifluoromethyl)-[1,1’-biphenyl]-3- yl)methyl)-2H-pyrido[1,2-a]pyrimidin-2-one (5e): Yellow solid; C22H13F5N2O; Yield = 70%; m.p. = 214-216°C. 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.20 (d, J = 7.3 Hz, 1H), 7.83-7.54 (m, 9H), 7.42 (d, J = 9.0 Hz, 1H), 6.80 (t, J = 7.2 Hz, 1H), 6.61 (s, 1H).13C NMR (75 MHz, CDCl3): δ (ppm) = 167.3 (CO), 153.3, 143.0, 141.5 (t, JCF = 29.1 Hz), 141.4, 136.3 (CH), 133.2 (t, JCF = 25.2 Hz), 131.0 (CH), 130.6 (q, JCF = 25.0 Hz), 130.4 (t, CH, JCF = 6.9 Hz), 130.3 (CH), 127.7 (2CH), 126.2 (CH), 126.1 (2CH), 125.7 (t, CH, JCF = 5.5 Hz), 124.8 (t, CH, JCF = 5.4 Hz), 124.2 (q, CF3, JCF = 270.6 Hz), 119.1 (t, CH, JCF = 5.0 Hz), 118.6 (t, CF2, JCF = 243.2 Hz), 113.4 (CH). 19F NMR (282 MHz, CDCl3): δ (ppm) = - 62.58 (CF3), -92.28 (CF2). HRMS(ESI): m/z [M+H] +calcd for C22H14F5N3O: 417.10208; found: 417.10357.
NMR Data: (Graphs 1-38)
Summary
In summary, we have provided a straightforward access to a new gem-difluorinated pyrido[1,2-a]pyrimidine-2-ones using a practical and genersal method. We first established a concise onepot strategy for the synthesis of gem-difluorinated pyrido[1,2-a] pyrimidin-2-ones by condensation of 2-aminopyridines with gemdifluorinated methyl propiolates. These compounds were then used as building blocks to synthesize a new 4-[(3-substitutedphenyl) difluoromethyl]-2H-pyrido[1,2-a]pyrimidin-2-ones via crosscoupling reactions. This valuable strategy is being applied to the production of a wide range of compounds that are currently under biological evaluation.
Acknowledgment
We would like to thank the Association of Specialization and Scientific Orientation (Lebanon) and Lebanese University for their support. We thank also the ‘Département d’analyses Chimiques et Médicales (Tours, France) for chemical analyses.
More BJSTR Articles: https://biomedres01.blogspot.com/





No comments:
Post a Comment
Note: Only a member of this blog may post a comment.