Therapeutic Applications of Coconut Water Mitigates Diabetes Mellitus in Oryctolagus cuniculus (Rabbit)
Introduction
Diabetes mellitus (DM) is an epidemic disease and causes severe problem to human health [1]. By definition, diabetes mellitus is a group of metabolic imbalances, results in hyperglycemia, which is either caused by abnormal production of insulin or its harmful physiology [2]. Diabetic patients are at more risk to cardiac, peripheral arterial and cerebrovascular diseases [2,3]. The chronic hyperglycemia of diabetes is associated with long-term damage, dysfunction, and failure of various organs including eyes, kidneys, nerves, heart, and blood vessels [4]. DM also disturbs function of liver, therefore, in blood serum the level of glutamic-oxaloacetic transaminase (SGOT) and Serum Glutamic Pyruvic Transaminase (SGPT) increases [5]. There is usually persistent thirst, polyuria, blurred vision, and weight loss followed by ketoacidosis or non– ketotic hyperosmolar condition that leads to stupor, coma and, if untreated causes death [6]. In 1993, the World Health Organization (WHO) Diabetes reporting group published global estimates for the prevalence of diabetes from 75 communities in 32 countries [7].
Worldwide, the prevalence rate of obesity, diabetes, and other metabolic syndromes is high [8,9]. There have been several previous estimates of the number of persons with diabetes. Prevalence of diabetes among adults (age 20–79 years) will increase to 7.7% (439 million) of the world population by 2030. This estimation is alarmingly high for developing countries where diabetes patients will increase to 69% by 2030 [10]. According to World Health Organization (WHO), diabetes will be the 7th leading cause of death in 2030 [11]. Currently, Pakistan occupies 7th position of high-risk diabetes-suffered countries [12]. The current prevalence of type 2 diabetes (T2D) in Pakistan is 11.77% [13]. Furthermore, according to National Survey (2016-17), 26% of Pakistan population is suffering from diabetes. Therefore, prevention of diabetes mellitus needs serious attention in health policy. By far the most popular approach to treat T2D is glycemic control in an attempt to reduce complications and death. In the past, several drugs for T2D including oral anti-diabetic agents (OAAs), insulin, and incretin-based drugs have been developed.
For instance, sulfonylurea-type drugs, insulin sensitizers such as TZDs (e.g., rosiglitazone and pioglitazone) and a biguanide, metformin, can directly lower insulin resistance and, subsequently, blood glucose. Glucose (re)absorption is viewed as an alternative way to lower blood glucose level. Inhibitors of Sglt 2 and glucosidase such asdapagliflozin, empagliflozin, and acarbose, inhibit the activity of sodium-glucose cotransporter-2 and glucosidases, respectively. Such inhibition leads to decrease glucose (re)absorption via the renal tubules and the intestine, respectively [14,15]. The available anti-diabetic drugs have limited rolesand cause serious sideeffects. For instance, metformin [16], pioglitazone [17], and SGLT-2 inhibitors [18] cause lactic acidosis, edema and heart failure, and dehydration, urinary and genital infections,respectively. Lowering blood glucose and lipid profiles by using natural products of plant origin as a possible therapeutic measure has become a promising scientific investigation. It has been reported that essential oils may be effective against type 2 diabetes mellitus. The daily consumption of olive oil had positive effect on fasting blood glucose and lipid profiles of healthy controls [19].
Experimental studies have shown that Hibiscus rosasinensis Linn. ethanol flower extracthas positive effect on blood glucose and lipid profile in streptozotocin induced diabetes in rats [20]. Aloe vera gel extract has hypoglycemic, hypotriglyceridemic properties in STZ-diabetic rats [21]. The natural Egyptian Morus alba root bark extracthas protective effects in diabetes by decreasing glucose level, moderate lipid peroxidation, and preservation of pancreatic β cell integrity [22]. CW is a rich source of essential metabolites including sugars, sugar alcohols, vitamin C,folic acid,free amino acids, phytohormones (auxin, cytokinin), enzymes (acid phosphatase, catalase, dehydrogenase, diastase, peroxidase, RNA -polymerases) and growth promoting factors [23]. Quantitative difference in the composition of the active components available in coconut water are 95.3 % water, 0.005% nitrogen, 0.56% Phosphoric acid, 0.25% Potassium, 0.69% Calcium oxide, 0.59% Magnesium oxide, 0.5% Iron, 0.8% reducing sugar and a total sugar of 2.08% [24]. Since, the coconut water has several medicinal properties such as hypocholesterolemic, antihypertensive, cardio protective, hepatoprotective, and hypolipidemic properties [25,26]. Therefore, the current study has evaluated the therapeutic potential of CW against the diabetic rabbits.
Materials and Methods
Selection of Animals
Oryctolagus cuniculus (Rabbit) was selected as experimental animals. Rabbits were purchased from local markets and divided into several groups on the basis of their body weights.
Induction of Diabetes Mellitus (DM)
Streptozotocin was used for the induction of diabetes mellitus in rabbits. The rabbits were made diabetic by injecting streptozotocin via peritoneum route [27]. Streptozotocin dose was administrated body weight dependent. To counteract initial hypoglycemia, glucose was given orally, also 10% glucose was provided in drinking water for next 24 hours [28].
Drug Administration
a. Group A was medicated with coconut water at the dose rate of 2.2g/kg body weight.
b. Group B was medicated with coconut water at the dose rate of 4.1g/kg body weight.
c. Group C was kept as null control without any treatment.
d. Group D was kept as negative control (diabetic but unmedicated).
e. Group E was considered as positive control and medicated with Glucophage (Metformin HCl) at the dose rate of 14 mg/kg body weight.
f. Drug administration was continued for regular 15 days after confirmation of diabetes mellitus.
Collection of Blood Samples
Blood samples were collected from all the groups with the following schedule; Day zero, day 03, day 08, day 13, and day 18. Blood samples were collected, stored in slant position and serum was oozed out upon clotting. Serum was collected in falcon tubes and was analyzed for glucose, triglyceride, cholesterol, HDL and LDL levels.
Analysis of biochemical parameters. Blood samples were analyzed for different parameters using their respective standard available kits (Semi Auto Chemistry Analyzer).
Statistical Analysis
The results obtained were analyzed statistically. Mean and standard deviation were determined. All the values of different groups were compared at different days using ANOVA. Online software, Prism, and Demo v05 were used for statistical analysis (www.graphpad.com).
Results
Induction of Diabetes Mellitus
All the rabbits of group A, B, D and E were intoxicated with streptozotocin (80mg/kg). Diabetes mellitus was confirmed on day 3rd of the experiment. Our results suggested that diabetic-induced rabbits lost their body weight (Table 1). Two groups (A and B) were treated with coconut water at dose rate of 2.2g/kg and 4.1g/ kg, while, one group (E) was medicated with 14mg/kg metformin HCl. Our results observed significant increase (P<0.0001) in the body weight of diabetic treated groups (Table 1). Upon treatment of diabetic rabbits with CW, it was observed that the treated rabbits were gaining body weight (Table 1). Conversely, significant decrease (P<0.0001) was observed in the body weight of positive control (Table 1). Our results observed that that coconut water and metformin increase the body weight in diabetic groups (Table 1).
Table 1: Time-scale analysis of body weight of rabbits (n=3).
Note: #M: Mean
$SD: Standard deviation
*CW: Coconut water
NC: Negative control
ΨPC: Positive control
Analysis of Blood Glucose Level
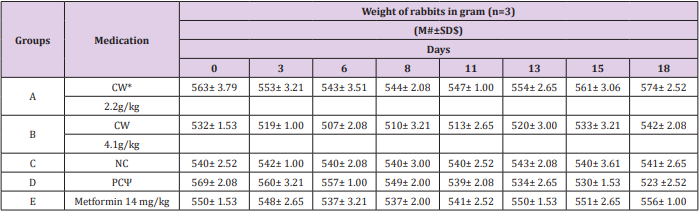
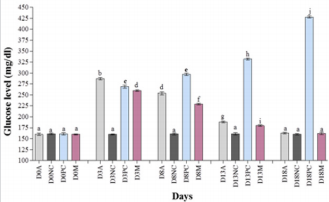
Blood glucose level of all rabbits was analyzed prior to exposure to STZ. The glucose levels were 160±2.52, 161±2.08, 161±2.08, 161±2.61, and 161±1.53 mg/dl of group A, B, C, D, and E, respectively (Figures 1 & 2). After intoxication with STZ (80mg/ kg), diabetes was further confirmed by blood glucose analysis. Blood glucose levels were found 287±3.00, 290±3.21, 269±3.61, and 260±2.00 mg/dl for group A, B, D, and E, respectively (Figures 1 & 2). Our results observed elevated glucose level in all the diabetic rabbits as compared to negative control (160±1.53mg/dl). Our findings suggested that administration of CW to diabetic groups at dose rate of 2.2g/kg and 4.1g/kg for eight days decreased the blood glucose level to 254±3.61 and 247±2.00 mg/dl, respectively. Moreover, the glucose level of metformin-treated group dropped down to 229±1.53 mg/dl. Furthermore, on day 13th and 18th, further decrease was observed in the glucose level of CW-treated groups A and B to 188±2.00 and 187±2.00 mg/dl and 163±1.53 and 162±1.53 mg/dl, respectively (Figures 1 & 2). Metformin also decreased the glucose level significantly. Hence, our findings demonstrated a significant difference (P<0.0001) in the blood glucose level of CW-treated and -untreated diabetic rabbits.
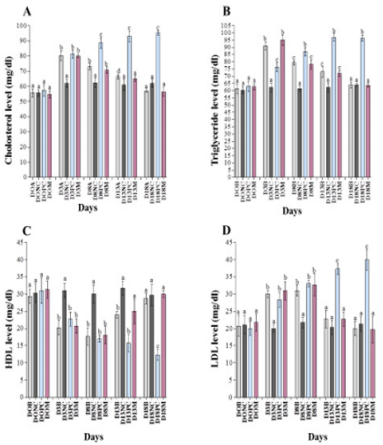
Analysis of Blood Cholesterol Level
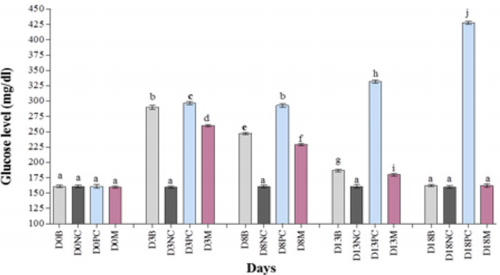
Before induction of diabetes, blood cholesterol level was determined and observed that group A, B, C, D, and E has blood cholesterol level of 55.0±2.65, 55.7±2.52, 57.7±2.08, 57.3±3.79, and 54.7±2.52 mg/dl, respectively (Figures 3A & 4A). Our results observed that upon induction of diabetes, cholesterol level was increased to 81.7±3.79, 80.3±3.21, 81.2±2.65, and 80.0±1.00 mg/dl as compared to negative control 62.0±2.65 mg/dl (Figures 3A & 4A) which show significant difference in all diabetic groups. Thereafter, the diabetic group A and B, were treated with CW at dose rate of 2.2g/ kg and 4.1g/kg, and group E were treated with metformin 14mg/kg. Our analysis of blood samples on day 8th observed that cholesterol level was reduced to 74.7±2.08, 73.0±2.00 and 70.7±2.08 mg/ dl in medicated group. Moreover, further decrease was observed at day 13th to 68.3±1.53 and 66.3±1.53 in the cholesterol level of CW-treated groups A and B and in metformin-administrated group 65.0±2.00 mg/dl. Further reduction was noted at day 18th in the cholesterol level to 58.0±1.00, 57.0±1.00, and 56.3±3.06 mg/dl of CW-treated groups A and B and metformin-administrated group. Therefore, we suggested that CW at the dose rate of 2.2 and 4.1 g/ kg leads to decrease in cholesterol level (Figures 3A & 4A).
Analysis of Triglyceride Level in Blood
Measurement of blood triglyceride (TG) level of healthy rabbits observed 62.0±2.00, 61.3±3.21, 60.3±2.52, 63.0±3.61, 62.7±2.08 mg/dl in group A, B, C, D, and E, respectively (Figures 3B & 4B). Analysis of STZ-induced diabetic rabbits confirmed that blood TG level was increased as compared to control. At day 3rd, the TG level of blood samples of diabetic rabbits increased to 90.3±3.21, 91.0±2.65, 76.3±3.21, and 95.0±3.61 mg/dl for groups A, B, D, and E, as compared to negative control 62.3±3.06 mg/dl for group C, respectively (Figures 3B & 4B). Treating diabetic rabbits, A and B with CW at the dose rate of 2.2 g/kg and 4.1 g/kg, and group E were treated with metformin at 14 mg/kg, significantly reduced TG level to 80.3±2.52, 79.3±1.53, and 78.3±3.06 mg/dl as compared to positive control 87.0±3.00 mg/dl at day 8th. With continuous treatment, TG levels were reduced to 74.3±2.08, 73.0±3.61 and 72.0±2.00 mg/ dl as compared to positive control96.7±2.52 mg/dl after blood analysis at day 13th. At day 18th, further reduction was noted in TG level (64.7±2.08, 64.0±1.00 and 63.7±1.53mg/dl) as compared to the day 13th as shown in Figures 3B & 4B. Therefore, it is concluded that CW at the dose rate of 2.2 and 4.1 g/kg decreases TG level.
Evaluation of High-Density Lipoprotein (HDL) in blood
Prior to diabetes induction, the blood high density lipoprotein (HDL) level of all the groups were measured as 31.3±1.53, 29.3±2.08, 30.3±1.53, 31.0±2.65, and 31.3±2.52 mg/dl for groups A, B, C, D, and E, respectively (Figures 3C & 4C). At day 3rd of diabetes induction, the HDL level was reduced to 20.3±2.08, 20.0±2.00, 22.7±2.08, and 20.7±2.08 mg/dl in diabetic group A, B, D, and E. The blood HDL level of negative control C (31.0±2.00 mg/dl) remained unchanged. Our results demonstrated that CW-treated rabbits (dose rate of 2.2 g/kg and 4.1 g/kg) showed elevated level of blood HDL of 18.7±2.52 and 17.7±2.52. Analysis of and 14 mg/ kg of metformin-treated samples obtained 18.0±2.65 mg/dl HDL concentration at day 8.Further increase was observed in HDL level in the groups treated either with CW or metformin. At day 13th and 18th, the HDL level was increased to 23.0±1.00 and 24.0±1.00, and 28.0±2.00 and 28.7±1.53 for diabetic groups treated with CW at the dose rate of 2.2g/kg and 4.1g/kg, respectively (Figures 3C & 4C). Consistently, the metformin-treated groups showed 23.0±1.00 and 28.0±2.00 mg/dl HDL concentration at day 13 and 18, respectively (Figures 3C & 4C). So, we recommend that CW increases the blood HDL level of diabetic rabbits (Figures 3C & 4C).
Evaluation of Low-Density Lipoprotein (LDL) in Blood
The healthy samples showed normal concentration of lowdensity lipoprotein (LDL) and obtained 20.0±3.00, 20.7±2.08, 21.0±2.65, 20.0±2.00, 21.7±2.52 mg/dl for groups A, B, C, D, and E, respectively. After induction of diabetes at day 3rd, the LDL level was increased to 28.0±1.00, 30.0±1.00, 28.3±2.08, and 31.0±2.65 mg/dl for groups A, B, D, and E, compare to negative control 20.0±1.00, respectively as (Figures 3D & 4D). Upon treatment with CW at the dose rate of 2.2g/kg and 4.1g/kg, group A and B showed slightly low LDL level of 29.3±1.53, 31.0±2.65, respectively. In parallel, metformin-treated diabetic group (E) at the dose rate of 14 mg/kg obtained 32.7±3.21 mg/dl. At day 8th, LDL level were decreased to 29.3±1.53, 31.0±2.65, 32.7±3.21 mg/dl, as compared to positive control 33.0±1.00 mg/dl. At day 13th, LDL levels were reduced in CW- as well as Metformin-treated groups to 24.3±2.52, 22.7±2.52, and 22.7±2.08 mg/dl, respectively. At day 18th, further decrease was recorded in LDL values in both the CW- as well as Metformin-treated groups to 21.3±2.08, 20.0±2.00, and 19.7±3.79 mg/dl, respectively. Conversely, the LDL level remained higher for positive control (40.0±3.00 mg/dl) and lower for negative control (21.3±2.08 mg/dl) as shown in Figure 3D & 4D. So, it is concluded that CW at the dose rate of 2.2g/kg and 4.1g/kg decreases LDL in diabetic rabbits (Figures 3D & 4D).
Discussion
Diabetes mellitus (DM) can mainly be attributed to the sedentary life style and calorie-rich diet. DM is linked with abnormal lipid metabolism and is considered as a major factor for the development of atherosclerosis and cardiovascular complication [29]. STZ causes hyperlipidemia along with hyperglycemic condition [21,30,31]. Previously, it was investigated that STZ causes diabetes by rapid depletion of beta-cells, which leads to reduction of insulin secretion [32]. In rats, intravenous injection of STZ (70 mg/kg of body weight) induces diabetes mellitus [33]. Moreover, Mozaffari et al., induced DM in rats by injecting STZ at dose rate of 90 mg/kg of the body weight [31]. In present study, STZ was used as DM inducer at the dose rate of 80 mg/kg body weight. Our results confirmed that STZadministrated rabbits lost their body weights, which is the ultimate symptom of diabetic rabbits [34-36]. After treatment with CW at dose rate of 2.2 g/kg and 4.1 g/kg, an increase occurred in the body weight of diabetic rabbits. Our results are in strong agreement of Preetha et al. and Saat et al. where they observed an increase the body weights of virgin coconut oil-treated diabetic rabbits [37,38].
Additionally, administration of STZ 80 mg/kg increased the glucose level of STZ-administrated rabbits. Several reports have investigated that STZ administration increases blood glucose level upon reduction of insulin secretion [34,39,40]. Our study follows Aly et al. when they induced hyperglycemia by injecting STZ at a dose rate of 65 mg/kg body weight [41]. In another study, STZ increased blood glucose level when injected a at dose rate of 60 mg/kg body weight [42]. Our results demonstrated that CW at the dose rate of 2.2 g/kg and 4.1 g/kg decreased blood glucose level of diabetic rabbits. Preetha et al. investigated that Albizia myriophylla and virgin coconut oil have significant hypoglycemic potential [37]. Additionally, a couple of studies have reported that mature coconut water and coconut kernel protein reduced the blood glucose level of diabetic rats [38,43]. Therefore, our study recommends that coconut water decreasedthe blood glucose level of diabetic rabbits. Our results observed elevated level of cholosterol in STZ-induced diabetic rabbits. Our results are in strong agreement that STZ accumulates high concentration of cholesterol diabetic rats [41,44].
We observed that CW reduced the blood cholesterol level of diabetic rabbits. Previously, palm kernel oil and coconut oil were used to reduce the total cholesterol levels of diabetic rats [45,46]. Our study also observed that diabetic rabbits were symptomized by elevated levels of triglycerides. Increase in triglyceride concentration is an essential indication of diabetic subjects [41,44,47]. Our study confirmed that CW caused a signifcant reduction in the concentration of triglyceride level of diabetic rabbits.Other studies have also reported that coconut oil and coconut water reduces triglyceride level and have anti-diabetic activities [45,46,48]. STZ administration decreases HDL levels in animals [30,44]. Our study observed that CWincreased HDL level in CW-treated rabbits. Same results werealsodocumented by several other reports [48- 50]. Finally, the present study investigated that STZ increased the LDL level STZ-intoxicated rabbits. STZ has been reported to cause a significant increase in LDL concentration which leads to DM [41,44,51]. Our study investigated that CW reduces LDL level in diabetic rabbits. other studies have also observed that coconut water was given to diabetic rats and same reduction in LDL level were found [48,52]. In another study, the synergistic effects of coconut oil and olive oil has demonstrated to reduce the LDL level [53]. Overall, our study demonstrated that CW has anti-diabetic potential and could be used as a nutraceutical or functional food for the management of diabetes and its associated complications.
Conclusion
STZ induced a characteristic multiphasic immediate response in rabbits. There was frequent urination, decreased physical activities and sluggishness in comparison to negative control. In addition, marked individual variations in response exist in terms of onset and severity of glycemic changes. The body weights were reduced in diabetic rabbits. There was also increase in cholesterol, triglyceride and low-density lipoprotein values in all the intoxicated rabbits with low values of high-density lipoprotein. After treatment with coconut water as well as metformin for two weeks, all the parameters became normal or near to normal when compared to positive control. Form the results of our study prove that coconut wateris effective against experimentally diabetic. The present work demonstrates that both concentrationsare capable of improving the diabetic complications. Conversely, coconut water showed an adverse effect in relation to the diabetic complications.
More BJSTR Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.