Triggered Cardiogenesis in Wingless 5a Treated Amniotic Mesenchymal Stromal Cells
Introduction
Adult cardiac myocytes are understood to be terminally differentiated. Although several reports have highlighted the presence of endogenous cardiac progenitor cells, an ongoing debate exits on their origin and role in myocardial regeneration [1,2]. Regardless, the millions of cardiac myocytes urgently required following a cardiac insult overwhelms their turnover rate and the myocardium commits to an adverse failure. To succeed a failing heart, substituting the demised cardiac myocytes with active ones becomes mandatory. Stem cell based cardiac regenerative therapy stands as one of the optional strategies examined. However, due to their multipotency, a direct inoculation of stem cells into a distorted cardiac micro-environment does not guarantee a cardiogenic commitment. Moreover, mounting evidence indicate a lack of in vivo trans differentiation into cardiac myocytes [3-7]. Thus, an in vitro commitment of stem cells to cardiac lineage specification is favored prior to their intramyocardial injection and should allow for better cardiac outcome.
In the course of cardiogenic development, an intricate and coordinated series of spatial and temporal changes occur governed by several signaling events such as the bone morphogenetic protein, the fibroblast growth factor, the Notch signaling, and the canonical and noncanonical Wnt signaling pathways. Extensive gain and loss-of-function studies have recognized the pivotal role of the Wnt secreted ligands in cardiac specification, morphogenesis, and differentiation [8,9]. The Wnt family consists of 19 known proteins in vertebrates. Its signaling is categorized into a canonical pathway involving the ß-catenin and noncanonical that utilize the Wnt/Ca2+ and the planar cell polarity pathways [8,9].
Of the noncanonical proteins, silencing the Wnt-5a glycoprotein has been implicated in a spectrum of abnormal cardiac development. The myocardium in the vertebrates originates from 2 subpopulation of progenitor cells located within a region of the mesoderm: the first and the second heart field [10]. The first heart field gives rise to the left ventricle and atria while the second heart field is located extra-cardially and its progenitors generate the right ventricle, the Outflow Tract (OFT), and sections of the atria [11]. Wnt-5a has been found to be specifically expressed in the second heart field progenitor cells in the caudal splanchnic mesoderm [12]. In conjunction with Wnt-11, Wnt-5a was shown to promote the development of second heart field cardiac progenitors [13].
Moreover, reports indicate a critical role of Wnt-5a in the morphogenesis of the OFT [12,14,15]. It is believed that Wnt5a orchestrates a temporal deployment and differentiation of progenitor cells from the second heart field to allow a enough elongation of the heart tube during the looping process. Impaired formation of the OFT leads to a spectrum of conotruncal defects such as overriding aorta, transposition of the great arteries, double outlet right ventricle, and persistent truncus arteriosus [16]. Notably, Wnt-5a transcription was also attenuated in T-box1 null mutant mice associated with OFT malformations in DiGeorge syndrome [17]. The cardiogenic effect of Wnt- 5a extends beyond embryogenesis as this ligand has been found to enhance the cardiac gene expression in endothelial progenitor cells and embryonic stem cells [18,19]. However, Wnt-5a cardiomyogenic potential on MSCs remains undetermined. In the current study, we sought to determine the cardiogenic potential of Wnt-5a on stem cells isolated from the amnion stroma of the human term placenta.
Materials and Methods
Amniotic Mesenchymal Stromal Cells
Human AMSCs were purchased from Scien Cell Research Laboratories at passage 1. The cells were isolated from the amnion stroma of the term placenta following the mechanical peeling of the amnion membrane from the fetal membranes. Cells were expanded and maintained in complete MSCM (Scien Cell Research Laboratories) as recommended by the provider and incubated in humidified 5% CO2 at 37°C. Cell passages from 4 to 7 were utilized in this study.
Gene Expression Profiling
AMSCs at 1.5×105 cells/ml were plated overnight in 6 well plates. The culture medium was then replaced with MSCM containing 1% Fetal Bovine Serum (FBS) and the cells were treated with Wnt-5a. Time-course and dose curve analyses were conducted in duplicates to determine an optimal dose and exposure time to Wnt-5a. The optimized condition was subsequently selected for further analysis. Total RNAs were isolated with the GENEzol™ TriRNA Pure Kit (Geneaid) and 1.5 µg of total RNAs were used to synthetize cDNA (iScript cDNA Syn Kit, Bio-rad). Quantitative PCR was performed using the QuantiFast SYBR Green PCR Kit (Qiagen). Data was then studied using the PrimePCR analysis software (Bio-rad). In brief, cardiac genes of interest were initially normalized to Nucleolin (NCL) then β-Myosin Heavy Chain (MYH7) gene expression by ΔΔCT protocol. The relative normalized gene expression was then calculated against the relative control group which is presented as relative normalized gene expression (fold) and regulation changes. In total, analysis of 5 cardiac genes was performed on Wnt-5a treated AMSCs and their sequences are presented.
Metabolic Activity
To determine whether Wnt-5a application could affect AMSCs viability, the metabolic activity of the AMSCs was evaluated following their exposure to Wnt-5a. Subsequently, an Alamar Blue assay (Fisher) was conducted as recommended by the manufacturer’s instructions. AMSCs at a concentration of 1.5×105 cells/ml were plated overnight in 6 well plates. Wnt-5a at 100ng/ ml was then applied for 3 days to AMSCs in 1% FBS MSCM (n=5). Untreated wells (n=5) were used as control. Reduction percentage and fluorescence intensity were then determined. Reduction percentage was calculated using the following formula:
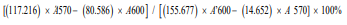
Where 117.216: molar extinction coefficient of Alamar Blue in the oxidized form at 600 nm 80.586: molar extinction coefficient of Alamar Blue in the oxidized form at 570 nm 14.652: molar extinction coefficient of Alamar Blue in the reduced form at 600 nm 155.677: molar extinction coefficient of Alamar Blue in the reduced form at 570 nm A570: absorbance of test wells at 570 nm
A600: absorbance of test wells at 600 nm
A’600: absorbance of negative control wells at 600 nm
A’570: absorbance of negative control wells at 570 nm
Immunofluorescence
AMSCs at 30,000 cells/well were cultured on cover slips and treated with Wnt-5a for 3 days at 100 ng/ml. Subsequently, the cells were fixed with 4% paraformaldehyde (Electron Microscopy Sciences) for 15 minutes, permeabilized with 0.5 % Triton X (Sigma), and then blocked with 3% bovine serum albumin (Fisher Scientific) in phosphate buffer for one hour. Overnight incubation at 4°C was conducted using primary antibodies for connexin 43 (1/1000, Abcam), GATA4 (1/200, Life Technologies), and SERCA2a (1/200, Life Technologies). Afterwards, fluorescent secondary anti-rabbit Alexa Fluor 488 (1/500, Life Technologies) was applied for one hour then treated with NucBlue Live ReadyProbes (Fisher Scientific) for nuclear labelling. Additionally, F-actin labelling was conducted using the ActinRed 555 ReadyProbes reagent (Fisher Scientific) in the AMSCs incubated with connexin 43 and SERCA2a. The coverslips were then collected and sealed on microscope slides in mounting media. Images were acquired using the Apotome.2 microscope and the LSM780 laser scanning confocal microscope (Carl Zeiss). For intensity quantification, images were randomly acquired at 40X magnification under a fixed exposure time. Analysis was completed using the CellProfiler imaging software.
Calcium Imaging
AMSCs at a density of 30,000 cell/well were plated in 27 mm Nunc glass bottom dishes (Thermo Fisher) and treated with Wnt5a at a concentration of 100 ng/ml for 3 days. Untreated AMSCs were used as control. The 1% FBS MSCM was then replaced with a serum free MSCM containing the cell permeable calcium probe Fluo-4/AM (5µM, Molecular Probes) and 20% Pluronic F-127 (P3000MP, Molecular Probes) in 1:1 volume ratio as recommended by the manufacturer. Afterwards, the cells were incubated with the Fluo-4/AM solution for 30 minutes at 37°C then washed twice with serum and Fluo-4/AM free MSCM followed by a de- esterification period of 30 min at room temperature. Untreated and Wnt-5a treated AMSCs were subsequently viewed using a LSM780 laser scanning confocal microscope (Carl Zeiss). Live image recordings were acquired in random fields (n=3 per group) to detect the intracellular calcium influx changes. For quantification, 5 cells/field (n=15 per group) were outlined as regions of interest in selected 3 to 5 Z-stack imaging planes.
Statistical Analysis
An unpaired t test was utilized to compare integrated intensities and Alamar Blue absorbance differences. One-way ANOVA was used to analyze Alamar Blue fluorescence intensity variations. P<0.05 was considered significant. Graphs were plotted using GraphPad Prism 7.
Results
Gene Expression Analysis
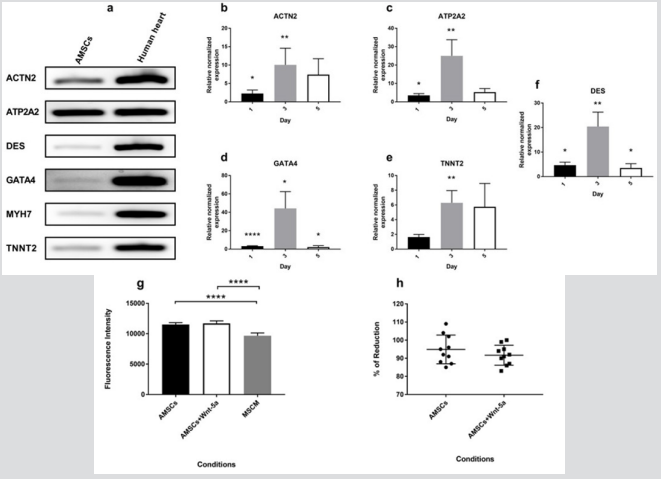
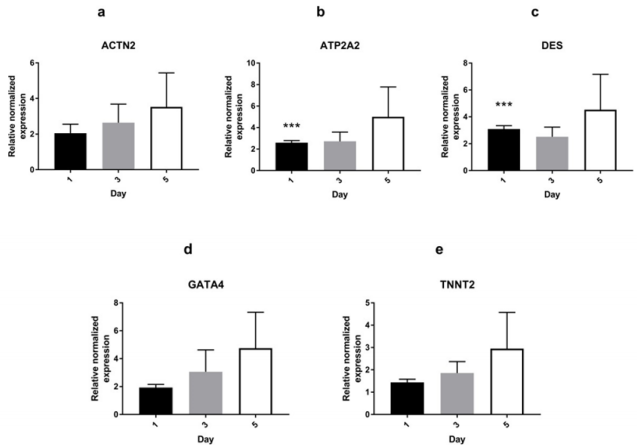
Untreated AMSCs showed a constitutive expression of a battery of cardiac-specific genes (Figure 1a). To determine whether Wnt5a triggers a cardiac lineage specification of AMSCs, these were exposed to a 7-day treatment of increasing Wnt-5a concentrations (supplementary Figure 1). Following optimization, AMSCs were then treated with 100ng/ml (n=4) or 200 ng/ml (n=2) of Wnt-5a and the total RNAs were collected on days 1, 3, or 5. At 200 ng/ ml, Wnt-5a treated AMSCs showed a significant upregulation of ATP2A2 (2.6 ± 0.2 fold; P<0.001) and DES (3.1±0.2 fold; P<0.001) 24 hours post-application. No significant upregulations of ACTN2, GATA4, and TNNT2 were observed on days 3 and 5 (supplementary Figure 2). In contrast, Wnt-5a at
Figure 1: mRNA expression of cardiac-specific genes in Wnt-5a treated AMSCs Non-treated AMSCs constitutively express cardiac genes (a). At a concentration of 100 ng/ml, Wnt-5a induced an enhanced cardiac genetic profile in AMSCs treated for 3 days. A significant increase in gene expression levels of ACTN2 (b), ATP2A2 (c), GATA4 (d), TNNT2 (e), and DES (f) was observed. Moreover, conditioning the AMSCs with Wnt-5a did not affect the cells’ viability and proliferation rates. This was determined by an Alamar Blue assay conducted after 3 days of exposure which revealed a similar fluorescence (g) and absorbance (h) intensities between the control and treated AMSCs (* P<0.05, ** P<0.01, *** P<0.001, **** P<0.0001).
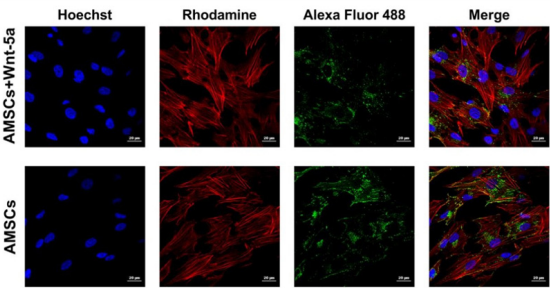
Figure 2: Connexin 43 cellular localization under confocal imaging Wnt-5a pre-conditioning of AMSCs did not seem to induce a major cellular displacement in connexin 43 protein. Clusters of this gap junction remained in the cytoplasm in both the treated and the untreated AMSCs.
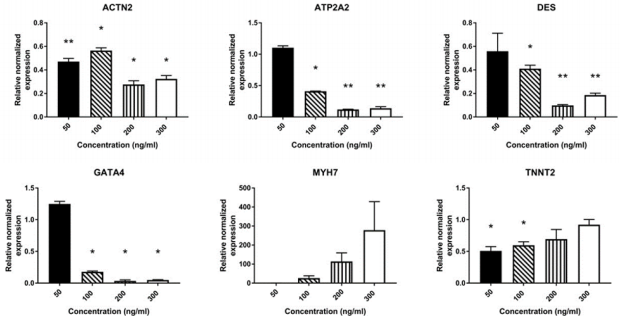
Supplementary Figure 1: mRNA expression of cardiac-related genes in AMSCs treated for 7 days with different concentrations of Wnt-5a AMSCs were treated with Wnt-5a at 4 different concentrations for 7 days: 50 ng/ml, 100 ng/ml, 200 ng/ml, and 300 ng/ml (n=2 per concentration). Wnt-5a was applied twice per condition on day 0 and day 3. A significant downregulation was observed in most of the expressed cardiac genes tested (* P<0.05, ** P<0.01).
Supplementary Figure 2: mRNA expression of cardiac-specific genes in AMSCs treated with 200 ng/ml of Wnt-5a AMSCs treated with Wnt-5a at 200 ng/ml exhibited significantly upregulated ATP2A2 (b) and DES (c) after 24 hours of exposure. There was no significant upregulation in ACTN2, GATA4, and TNNT2 genes (*** P<0.001).
100ng/ml enhanced the cardiac genetic profile of the AMSCs. Notably, a significant upregulation of a variety of cardiac genes were observed following a 3-day exposure to Wnt-5a (Figure 1b1f). Wnt-5a treated AMSCs exhibited a significant increase in gene expression of ACTN2 (10.1 ± 4.5-fold; P<0.01), ATP2A2 (25 ± 8.8 fold; P<0.01), DES (20.4 ± 5.9 fold; P<0.01), GATA4 (44.2 ±18.3 fold; P<0.05), and TNNT2 (6.3 ± 1.6 fold; P<0.01). Hence, a single application of Wnt-5a at 100 ng/ml to AMSCs for 3 days was chosen as the optimal condition to trigger an upregulation of cardiac genes.
Alamar Blue
The metabolic activity of AMSCs was determined following their treatment with Wnt-5a at 100 ng/ml for 3 days. Absorbance and fluorescent intensities were calculated. Treating the AMSCs with Wnt-5a did not affect their proliferation rates nor their survivability. The Alamar Blue assay revealed a non-significant difference in the fluorescence intensity (Figure 1g) and the percentage of reduction (Figure 1h) between the untreated and the Wnt-5a treated AMSCs (P=0.3 for absorbance and P=0.57 for fluorescence).
Immunofluorescence
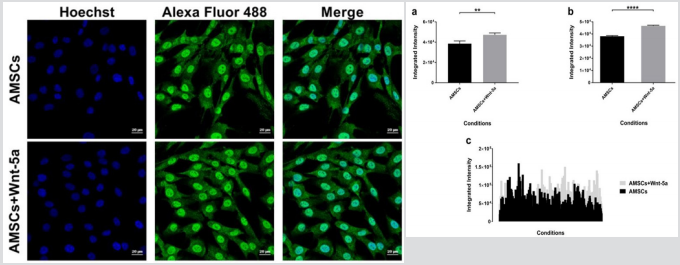
To determine whether the upregulation in cardiac-specific genes was correlated with an increased protein expression, Wnt-5a treated AMSCs were fixed and underwent an immunofluorescence staining for connexin 43, GATA4, and SERCA2a. In contrast to our reported data, cellular imaging revealed no significant changes in the connexin 43 localization (Figure 2). The protein was spotted in the peri-nuclear regions and on the cell membrane whereas Wnt5a treatment did not seem to induce a shift towards a membranal prevalence. GATA4
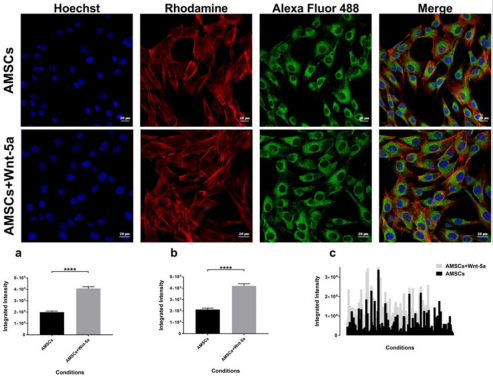
protein expression was detected in the untreated AMSCs. Nonetheless, treatment with Wnt-5a enhanced its nuclear expression (Figure 3). Under similar imaging conditions, AMSCs exposed to Wnt-5a showed a significant and increased integrated intensity when compared to the untreated cells (3.8×104 ± 581 pixels per nucleus in untreated AMSCs vs 4.70×104 ± 524 pixels in treated AMSCs; P<0.0001). Likewise, SERCA2a was observed in the untreated AMSCs but its cytoplasmic expression increased following Wnt-5a treatment. Image analysis revealed a substantial gain in the SERCA2a integrated intensity in the cells exposed to Wnt-5a (Figure 4); 1.9×105 ± 104 pixels per cytoplasm in untreated AMSCs vs 4.1×105 ± 1.4×104 pixels in treated AMSCs; P<0.0001).
Figure 3: Connexin 43 cellular localization under confocal imaging Wnt-5a pre-conditioning of AMSCs did not seem to induce a major cellular displacement in connexin 43 protein. Clusters of this gap junction remained in the cytoplasm in both the treated and the untreated AMSCs.
Figure 4: SERCA2a intensity analysis SERCA2a protein expression in Wnt-5a treated AMSCs and control cells. Charts showing a significant difference in the integrated intensity per image (a), per nuclei (b), and in a data plot summary (c) (**** P<0.0001).
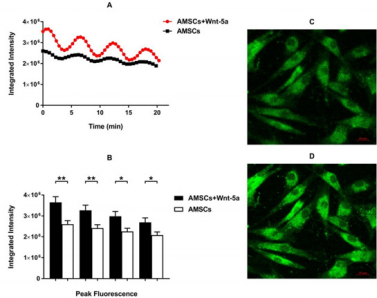
To further elucidate whether the upregulation of SERCA2a enhanced the intracellular calcium reuptake by the reticulum endoplasmic, Fluo-4/AM was loaded into Wnt-5a treated AMSCs and observed by confocal microscopy. Time series acquisition revealed a spontaneous and synchronized sinusoidal pattern in the Wnt-5a treated and untreated AMSCs (supplementary video). This pattern was mostly evident in specific Z-stack images across groups. Notably, quantitative analysis of selected Z-stack planes with a sinusoidal pattern showed a significant enhancement in the intracellular calcium level in the Wnt-5a treated AMSCs compared to control cells (Figure 5A). The rhythmical upregulation of calcium signaling fell in agreement with a peak intensity assessment which significantly increased in the Wnt-5a treated AMSCs (Figure 5B; 2.6×106 ± 1.7×105 pixels in untreated AMSCs vs 3.6×106 ± 2.7×105 pixels in treated AMSCs at the highest peak; P=0.0015).
Figure 5: Calcium imaging assessment Spontaneous synchronized and rhythmical sinusoidal pattern can be observed in the untreated AMSCs which was more pronounced and prominent following Wnt-5a treatment (A). Peak intensity evaluation showing a significant increase in the AMSCs conditioned with Wnt-5a in the multiple peaks detected (B). Representative images of the time series labelling the intra-cellular calcium flow in low (C) and high (D) intensity (* P<0.05, ** P<0.01).
Discussion
The cell cycle arrest halting the division of cardiac myocytes is the underlying cause to all heart disease abnormalities. Recent evidence has shown that cardiac myocytes of neonatal mice can regenerate after apical resection of the myocardium [20]. Such findings were however questioned in further reports [20,21]. Hence, considering that the mechanisms to induce mitosis in endogenous cardiac myocytes are yet undetermined, the in vitro cardiogenic trans differentiation remains an ideal model to further explore. The AMSCs are stem cells isolated from the amnion membrane of the human term placenta. These cells are of fetal origins with immune suppressive properties and constitutively express several cardiac markers as well as embryonic stem cell and MSCs markers [22,23]. In this regard, we have confirmed the inherent cardiac gene expression since our untreated AMSCs expressed a spectrum of cardiac markers such as desmin, troponin T, GATA4, and actinin (Figure 1a).
The Wnt signaling ligands of the canonical and noncanonical pathways play an essential role in Cardiogenesis during embryonic development [8,9]. Although Wnt-5a has been shown to play an central role in cardiac development, its cardiogenic effects have been scarcely identified in the MSCs population. The metabolic activity of AMSCs exposed to Wnt-5a was not altered and did not induce a change in the AMSCs proliferation rates. Of the upregulated cardiac genes, a remarkable fold increase of GATA4 and ATP2A2 can be distinguished. Similar to Wnt-5a, GATA4 plays an essential role in cardiac development. It is one of the early transcription regulators detected during Cardiogenesis that is even expressed prior to the formation of the linear heart tube [24,25]. GATA4 was also shown to interact with the Wnt pathway during Cardiogenesis and directly regulate the gene expression of Wnt-11 [26]. Silencing GATA4 leads to the formation of congenital heart defects while genetic mutations can be responsible for multiple congenital heart disorders [27,28]. Herein, GATA4 gene expression correlated with an over-expression of the GATA4 protein. Moreover, as a transcription factor, GATA4 was shown to induce the upregulation of other cardiac genes [29].
Accordingly, the enhanced amplification observed in the investigated cardiac genes could be attributed to the initial upregulation of GATA4 which could then potentiate their expression. Wnt-5a in treated AMSCs significantly upregulated ATP2A2 gene expression and its encoding SERCA2a protein. SERCA2a pump is the main regulator of calcium homeostasis in the functioning cardiac myocytes [15]. SERCA2a aberrant activity leads to a reduced calcium reuptake by the sarcoplasmic reticulum at the end diastole, abnormal relaxation, decreased actin myosin coupling, and an adverse contraction in the ensuing systole [30]. Gene therapy employing the injection of SERCA2a in a viral vector has yielded an improvement in cardiac performance in small and large animal models as well as in clinical trials [31,32]. More importantly, the upregulation of ATPA2A2 and its encoding SERCA2a protein in this study was in accordance with the increase in the intracellular calcium signaling observed under live imaging.
Untreated AMSCs presented a primitive and rudimentary calcium flow which developed into an enhanced and prominent pattern following Wnt-5a treatment. The Wnt signaling ligands exert their biological activity by triggering several downstream pathways. Wnt-5a of the noncanonical family was shown to activate the protein kinase c and calcium/calmodulin pathway [8,9] which could additionally justify the increased calcium flow detected in the treated AMSCs. Wnt-5a exposure did not alter the expression of all the cardiac proteins we investigated. In contrast to our reported data [33], much of the connexin 43 remained concentrated in the cytoplasm in the treated AMSCs. This could be due to differences in the cell isolation techniques and conditioning procedures. This study has focused on investigating the effects of Wnt-5a on a subgroup of cardiac genes. The broader effect of Wnt-5a on AMSCs could be investigated using microarray analysis.
Moreover, we determined here the cardiogenic impact of Wnt5a on AMSCs. Since it’s been shown that Wnt-11 and Wnt-5a interact during cardiac development, co-applying these 2 ligands to AMSCs could yield an increased and synergistic cardiogenic potential. In conclusion, we have demonstrated that a temporal application of Wnt-5a at a concentration of 100 ng/ml can significantly upregulate the expression of a spectrum of cardiac genes in AMSCs without causing any toxic effects on the cells’ metabolic activity. This gene upregulation correlated with an enhanced expression of cardiac proteins that play a key role in cardiogenesis such as GATA4 and SERCA2a. In parallel, an enhancement in calcium flow could be detected reflecting a pronounced contractile activity. Thus, we believe that AMSCs treated with Wnt-5a can be a potential substitute of the cardiac myocytes lost at the myocardial injury site [34-36].
Acknowledgment
This work was supported by a research grant from the Richard and Edith Strauss Foundation. Dr. Schwertani is supported by CIHR and NSERC. The authors would like to thank the molecular imaging platform at the Research Institute of the McGill University Health Center.
More BJSTR Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.