A case of Linezolid-Associated Hyponatremia in a Saudi Patient
Introduction
Linezolid is an oxazolidinone antibacterial agent that inhibits bacterial reproduction by selective binding to a site on the 23S ribosomal RNA of the 50S subunit, thereby preventing initiation complex formation with the 70S ribosomal subunit. It is bacteriostatic against enterococci and staphylococci, and bactericidal for a majority of streptococci isolates. It has a clinically important role in the treatment of multidrugresistant pathogens, such as methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant enterococci[1].
Linezolid is generally well tolerated with the most commonly reported adverse effects being headache, metallic taste, dizziness, diarrhea, nausea, vomiting, elevated liver enzymes, increased Blood Urea Nitrogen (BUN), thrombocytopenia, pruritus and rash[2].
Syndrome of inappropriate antidiuretic hormone secretion (SIADH) also called ‘Schwartz- Bartter syndrome’ is a disorder of impaired water excretion caused by the inability to suppress the secretion of antidiuretic hormone (ADH)[3]. If water intake exceeds the reduced urine output, the ensuing water retention leads to the development of hyponatremia. It accounts for about 1/3 of all cases of hyponatremia. Hyponatremia is the unbalance between total body water and sodium defined as a serum sodium concentration of <135 mEq/L; it occurs in up to 8% of the general ambulatory population and 15% to 30% of hospitalized patients. Population at risk of hyponatremia include infants, hospitalized patients (especially children and critically ill patients), adults > 65 years of age, individuals with neurologic or mental impairment, and surgical patients. Risk factors for hyponatremia include: exercising in a hot environment, use of the illicit drug ecstasy, fever, vomiting, diarrhea, burn injury, excessive ingestion of water, and receiving IV hypotonic fluids or plain dextrose 5% in water (D5W)[4].
There are certain medications with well-known mechanism to cause hyponatremia and can affect the sodium and water homeostasis such as diuretics, or affect water homeostasis via increasing hypothalamic production of ADH such as antidepressants[5]. Hyponatremia is not a labeled adverse drug reaction with linezolid, our search of the literature identified three case reports and a retrospective cohort study that reported the incidence of hyponatremia in a cohort of 61 Japanese patients receiving linezolid[6,7,8,9].
Case Report
An 81-year old male was admitted as a case of aspiration pneumonia and developed a urinary tract infection during admission. He was bedridden, with a known history of diabetes mellitus, hypertension, grade 1 diastolic dysfunction, hypothyroidism, Parkinson’s disease and a history of diabetic-foot amputation. During his admission due to a presumed urinary tract infection, urine culture was requested and results showed MRSA growth. Linezolid treatment 600 mg intravenous infusion every 12 hours was initiated.
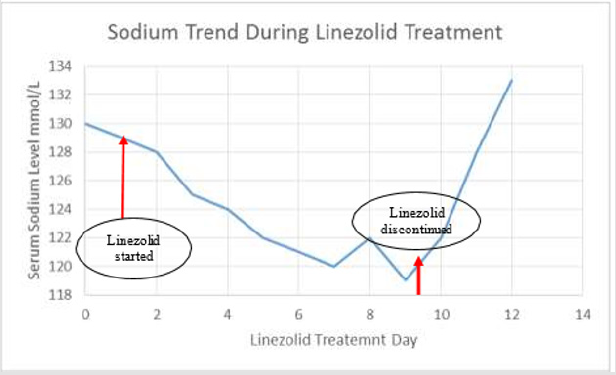
The patient’s sodium level dropped gradually to a level of 119 mEq/L on day 8 of linezolid treatment, from a level of 130 mmol/L prior to initiating linezolid. The patient’s sodium levels are shown in (Figure 1).
At the time of treatment, his estimated glomerular filtration rate (eGFR) was 92. On day 7 of treatment: plasma osmolality was 261 mOsm/kg, and urine osmolality was 198 mOsm/kg. On day 9 linezolid was discontinued and levels returned to normal within 2days.
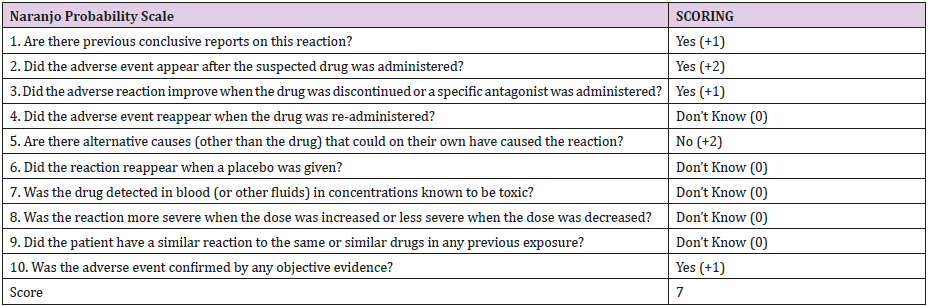
Causality Assessment: using the Naranjo Adverse Drug Reaction Scale, linezolid had a score of (7) (Table 1), suggesting that linezolid as a cause of SIADH adverse drug reaction is probable.
Table 1: Naranjo Adverse Drug Reaction Score Assessing Causality of Hyponatremia with Linezolid in Our Patient.
Discussion
his is the first case to our knowledge describing linezolidinduced hyponatremia in a Saudi patient. Linezolid is the drug of choice in treating patients for MRSA infections especially when vancomycin treatment failure is suspected. In addition, Linezolid can be continued when discharge is desirable due to the availability of linezolid in an oral dosage form. This makes the risk of hyponatremia more problematic as close monitoring of sodium levels may not be applicable. Hyponatremia if sever can result in hospital readmission, contribute to major comorbidities such as cerebral edema, cardiopulmonary arrest, seizure, and coma.
In the retrospective cohort study by Tanaka et al of 61 Japanese patient receiving linezolid hypernatremia was observed in 11 (18%), and severe hyponatremia was observed in 1 (2%), where the frequency of the development of hyponatremia was also significantly higher in patients who received a combination of a potassium sparing diuretic and linezolid[9].
Likewise, our patient was also on a diuretic; however on a loop diuretic (bumetanide) not a potassium sparing diuretic. As diuretics are well known to cause hypoonatremia on their own it alone could not explain the observation of the sudden drop post initiation of linezolid in the study by Tanaka et al in addition to the other case reports identified[6,7,8].Together with the improvement and rise in sodium levels upon discontinuation of linezolid described in the reported cases increase the likelihood of association.
There is currently no labeled drug-drug interaction between linezolid and any of the diuretics prompting the need to monitor for signs and symptoms of hyponatremia. We recommend that sodium levels be monitored in patients treated with linezolid and to suspect it as a culprit in patients who develop unexplainable hyponatremia. We also recommend creating a drug-drug interaction alert in health information systems to alert about the possible risk of hyponatremia in patients receiving concomitantly a diuretic and linezolid.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.