Causes of the Exceptional Emissions in the Conditions of Compliance of Technological Regulations
Introduction
In the conditions of industrial production, compliance with technological regulations does not always ensure the operation of equipment in the legal requirements [1]. Even with compliance with all requirements of technological regulations, unauthorized environmental contamination is possible.
Analysis of Literary Data and Statement of the Problem
Despite the absence of deviations from the regulations approved at the enterprise during the sintering process, gases with an increased content of carbon monoxide in the off-gas were released into the atmosphere [2].
The Purpose and Objectives of the Study
The purpose of the research was to determine the causes of excess emissions of CO in the waste gases, with full compliance with technological regulations [3].
Materials and Methods of Research
To investigate the causes of pollution of the air basin by overstandard emissions, the following documents were analyzed: certificates of compliance with the requirements of environmental legislation in the field of atmospheric air protection; acts of sampling emissions from stationary sources; protocols measuring the content of pollutants into organized emissions from stationary sources; technological instruction for the production of fluxed blast-furnace sinter; regulations of the dosing department of sintering plant [4,5].
Research Results
Within the agglomeration process, a number of sequentially parallel oxidation-reduction reactions occur, including those related to the formation of carbon monoxide (II) - CO. The speed and quantity of the produced CO is determined by the kinetic and thermodynamic conditions of the processes. The basis for the formation of carbon monoxide (II) is the following process
СO + О2 → CO2, k2
The speed of each stage of this series-parallel process (sequential-along carbon, parallel-in oxygen) is determined by the rate constants k1 and k2, as well as by the concentrations of the initial, final and intermediate substances [6]. Thus, the emission of carbon monoxide can be influenced by the kinetics (rate of reaction) of the process and the conditions of thermomass exchange in the unit. However, said oxidation of carbon to carbon monoxide (II) and carbon monoxide (IV) are reversible and equilibrium, i.e., flowing both in a straight line
and in the opposite:
As for all equilibrium reactions, the degree of conversion is determined by the isothermal potential Δ G and, respectively, by the equilibrium constants of each stage k1 and k2:
Δ G = - RT lnK = - RT ln (kпр/kобр)
In turn, the thermodynamic parameters are also related to the process temperature:
The formation of an intermediate product of CO depends on the kinetic and thermodynamic parameters of the reaction. In addition, as will be shown below, there are also external influences on the process [7,8]. The thermodynamics of the reduction of carbon dioxide is well studied. The reaction C + CO2 → 2CO has an enthalpy of 163 kJ/kmol, is endothermic and proceeds with heat absorption. The equilibrium composition of the gas phase shows that at a temperature below 500 °C it consists mainly of CO2, and at a temperature of 1000 °C CO2 is almost completely reduced to CO. The heterogeneous reaction of C + CO2 is multistage. Due to the collision of atoms and molecules with vacant sites and due to valence forces, intermediate complexes are formed at the active sites of the surface. Molecules on the active centers of the carbon surface dissociate, and chemisorptions of atoms occurs, rather than molecules [9,10].
Discussion of Results
In the case under consideration, external factors such as the presence of moisture in the charge, the use of fines, the use of noncalcined starting materials could be the causes of excess emissions. Fine fraction. The reaction for the formation of carbon monoxide (II) С → CO → CO2 occurs both in the gas phase and at the interface. Therefore, the overall process speed depends critically on the mass exchange conditions in agglomeration conditions. Depending on the speed of the chemical reaction, several process regimes are distinguished. At low temperatures, the rate of chemical reaction of the fuel with the oxidizer is small and is a limiting process in the overall process. A constant concentration of oxidant is maintained in the pores of carbon. The speed is described by the Arrhenius equation. The experimentally obtained values of the activation energy are: for the reactions of carbon oxidation with air, 168 kJ/ kmol, for carbonic acid reduction of 361 kJ/kmol. With increasing temperature, the rate of chemical reaction increases much more than the growth of diffusion occurs and the gasification process begins to be limited by the supply of gas into the particle.
At the same time, the concentration of CO2 decreases
towards
the center of the particle. At high temperatures, the oxidation of
the fuel particle is no longer limited by the speed of the chemical
reaction or by the consumption of the oxidant inside the particle, but
mainly by the diffusion of the oxidant through the boundary
layer surrounding the particle. The concentration of the oxidant on
the outer surface is thus small. The process is entirely determined
only by the hydrodynamic conditions on the external. The oxidation
regime is diffusion, and is described by the criterial equations of
the mass transfer 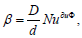 . For small particles at Nuдиф = const,
the process speed is inversely proportional to the square of the
particle diameter. Studies of the processes of carbon oxidation
and CO2 reduction have shown that in a purely kinetic region the
reactions proceed in a very complex order varying from 0 to 1. As
the temperature increases, the macrokinetic order of the reactions
increases, approaching the first. At 1150-1200 oC, the actual
chemical order is practically close to the first.
. For small particles at Nuдиф = const,
the process speed is inversely proportional to the square of the
particle diameter. Studies of the processes of carbon oxidation
and CO2 reduction have shown that in a purely kinetic region the
reactions proceed in a very complex order varying from 0 to 1. As
the temperature increases, the macrokinetic order of the reactions
increases, approaching the first. At 1150-1200 oC, the actual
chemical order is practically close to the first.
Experimental data showed that even at very low temperatures, 300-500 oC, the reaction with CO2 occurs only on the outer surface of the particles. As the diameter of the particles increases, the CO yield decreases and a dependence on the coal grade is revealed. Thus, reducing the particle size of both carbonaceous materials and other components of the charge results in an increase in CO content in the off-gas. Presence of moisture. Despite the fact that moisture is always present in the initial mixture for agglomeration, the excess of its content in the charge has a negative effect on the process. This is due to several factors. For the evaporation of water, additional energy is required, which leads to a lower process temperature or requires an additional amount of carbon. A change in temperature leads both to a change in the kinetic parameters of the process and to the processes of mass transfer. In addition, the presence of water leads to an additional increase in CO concentration in the gas phase. During the interaction of water vapor with the carbon surface
From the stoichiometry of this reaction, it is easy to calculate that an increase in the water content per 1 kg leads to the formation of 1.2 m3 of CO in the gas phase. Non-calcined carbonate. The introduction of carbonates into the reaction mixture instead of oxides leads to several negative consequences. The decomposition of carbonates requires additional energy, which leads to a lower process temperature or requires an additional amount of carbon. A change in temperature leads both to a change in the kinetic parameters of the process and to the processes of mass transfer. However, a much more significant factor is that when carbonate decomposes carbon dioxide is released: CaCO3 → C aO + CO2. The appearance of an additional amount of CO2 in the gas phase leads to a shift in equilibrium in reversible reactions: С → CO → CO2; СО2 → CO → C. From the stoichiometry of these reactions, it is easy to calculate that an increase in the content of uncalculated carbonate per 1 kg leads to the formation of 0.2 m3 of CO in the gas phase.
Conclusion
a) Reducing the size of particles, both carbon-containing materials and other components of the charge, leads to an increase in the CO content in the off-gases
b) An increase in the water content per 1 kg leads to the formation of 1.2 m3 of CO in the gas phase
c) An increase in the content of non-calcined carbonate per 1 kg leads to the formation of 0.2 m3 of CO in the gas phase.
Annotation
The reasons causing excessive CO emissions due to such external factors as the presence of excessive moisture in the charge, the use of fines, the use of non-calcined starting materials is considered. The mechanisms of formation of a large amount of CO are considered, methods and technologies for preventing emergency processes are proposed. It is shown that a decrease in particle size leads to an increase in CO emissions; an increase in the water content per 1 kg leads to the formation of 1.2 m3 of CO in the gas phase; an increase in the content of non-calcined carbonate per 1 kg leads to the formation of 0.2 m3 of CO in the gas phase.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.