Discovery of Large Quantity of Acanthocytes in Ovarian Follicular Fluids of the Infertility Patients
Introduction
The prevalence of human infertility has been increasing. With the application of in Vitro Fertilization (IVF) technologies, approximately 37% of patients can achieve pregnancies on their first attempt [1]. However, the mechanisms of infertility, especially in the category of endometriosis and the unexplained, still need extensive studies. IVF patients receive large amount of follicular stimulating hormones for the purpose of producing more than one egg. After hormonal treatments, the patients undergo a procedure of follicular fluid aspiration which allows embryologists to collect the eggs from these fluids. Our initial hypothesis was that the presence of immune cells in the follicular fluids may be related to the infertility. Therefore, we observed the cells present in the follicular fluids of over 200 patients. A surprise common feature appeared in these fluids are the star-shape cells. Our focus was redirected to these cells. They varied in sizes and some of them are smaller than the red cells. There has never been a report of these cells being present in the ovarian follicular fluids. Due to the presence of red blood cells in follicular aspirates, it is technically difficult to isolate these star-shape cells out of follicular fluids. After many failed attempts, we successfully obtained these cells by using two centrifugation speeds. Apparently, these cells were largely ignored in the past. In this study, by using the scanning electron microscope we further confirmed that these cells are acanthocytes.
Materials and Methods
The research proposal was approved by the Johns Hopkins Medicine Institutional Review Board (IRB study review number: IRB00185243) as an exempted category due to the pathological wastes were used for the study. For this type of study formal consent is not required. The follicular aspirates from the patients that underwent in vitro fertilization procedures were collected in the petri dishes immediately after the eggs were collected for the patients. The samples are deidentified except for the diagnoses. The cells were then observed under inverted microscope. Pictures of the cells in the follicular fluids from more than 200 IVF patients were taken for the future analyses.
Sample preparation for Scanning Electron Microscopy observation
The follicular fluids were centrifuged at 500G for 10 min at room temperature. The supernatant was collected and centrifuged at a higher speed (1750G) for 10 min at room temperature. The pellets were washed with D-PBS and then fixed in 2% paraformaldehyde, 2% glutaraldehyde, 100 mM sodium cacodylate, 3 mM MgCl2 and placed on coverslips in a 6 well plate. Following overnight fixation at 4oC, samples were rinsed in 50 mM cacodylate buffer containing 3 mM MgCl2 and 2.5% sucrose, and then post-fixed with 1% osmium tetroxide in cacodylate buffer for 1 hour on ice in the dark. Samples were then rinsed in dH2O and dehydrated through a graded series of ethanol, and transferred to ethanol: HMDS (Hexamethyldisiloxazne Polysciences), 1:1 solution, followed by pure HMDS. Samples were then dried in a desiccator overnight. The coverslips were attached to aluminum stubs via carbon sticky tabs (Pella) and coated with 40 nm of AuPd using a Denton Vacuum Desk III sputter coater. Stubs were viewed on a LEO1530 FE Scanning Electron Microscope.
Results
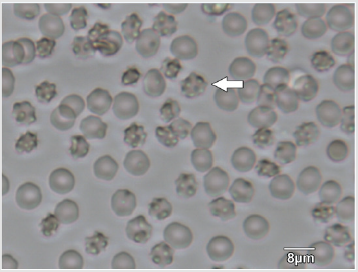
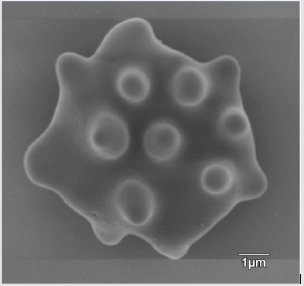
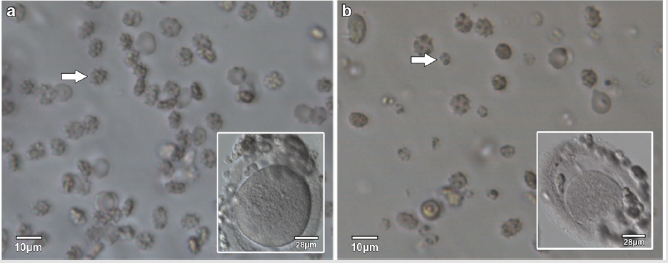
The appearance of the star-shaped cells in the follicular fluids is shown in Figure 1. These cells were isolated and prepared for the scanning electron microscopy observation. In the Figure 2, it showed fine structures of these star-shaped cells which possess same morphology as described by Lessin et al. [2]. They were called acanthocytes. The patients with endometriosis showed consistent high proportion of acanthocytes from 95% to 100% in the follicles containing chocolate color fluid (moderate endometriosis, Figure 3a), with great amount of fragmented acanthocytes seen in darker chocolate fluid (severe endometriosis, Figure 3b). The oocyte (Figure 3a) from the fluid with the moderate endometriosis showed grainy cytoplasm whereas the oocyte from the fluid with severe endometriosis presented a significant degenerated morphology (Figure 3b).
Figure 1: Star-shape cells (arrow) in follicular fluids of patients underwent treatment of controlled ovarian stimulation for the In vitro fertilization procedure.
Figure 3: Acanthocytes and oocytes from follicular fluids with moderate endometriosis and severe endometriosis.
a) Acanthocytes (arrow) from follicular fluid with moderate endometriosis, with the oocyte (insert) from the same follicular fluid.
b) Acanthocytes and fragmented acanthocytes (arrow) from the follicular fluid with severe endometriosis, with the oocyte (insert) from the same follicular fluid.
Discussion
Acanthocytes, also called “Spur cell”, have thorn-like projections, irregularly distributed over the surface of red cells. Acanthocytes are found in the blood of patients in variable proportions of between 5 and 50% of red cell populations (Warner, 2009). These acanthocytes are associate with many diseases such as abetalipoproteinemia, alcoholic liver disease, postsplenectomy state and malabsorptive states [2]. We, for the first time, demonstrated that acanthocytes are associated with infertile patients who are undergoing in vitro fertilization procedures, especially in the patients diagnosed with endometriosis.
Endometriosis is defined as chronic disorder with presence of endometrial-type mucosa outside the uterine cavity [3]. The estimated prevalence of endometriosis ranges from 2% to 15% of all reproductive aged women [4]. The precise etiopathogenesis of endometriosis is still unknown. Extensive studies suggest that genetic, hormonal, immunological and inflammatory processes are involved in the development of this condition [5]. The immune cells, such as mast cells [6] and macrophage [7], are indeed increased at endometriotic site. The serum hematological parameters were analyzed in patients with endometriosis [8] but acanthocytes were not mentioned in that study. In addition, endometriosis is associated with an increase in systemic oxidative stress, affecting the antioxidative defenses of circulating erythrocytes [7].
The mechanisms of the presence of acanthocytes in the aspirated follicular fluids will need further studies. With the blood vessels distributed around ovarian follicles, it is easily understood that the blood cells would be present in the follicular fluids when being punctured during the aspiration procedure. However, the varied amount of acanthocytes among the infertile patients (ranging from 5% to 100%) could be associated with underlying causes of infertility which have not been realized or diagnosed. Levin et al. [9] reported erythrocyte aggregation in peripheral venous blood was largely present in the patients with ovarian hyperstimulation syndrome indicating a capillary leakage. The question that needs to be asked is, did these patients become infertile due to the primary abnormal vascular structures? Or did the vascular structures become damaged by the exogenous hormonal treatments? Clearly, the vascular damage could be the true etiology that causes abnormal microenvironment resulting in dysfunctional follicular development. The competence of oocytes inside these follicles is jeopardized. Our results support this notion that oocyte from follicular fluids with moderate endometriosis showed better morphology whereas the oocyte from severe endometriosis became degenerated. The acanthocytes in the follicular fluids with severe endometriosis were fragmented. These results indicated that the treatments for some infertile patients should focus on improving the vascular circulation and ovarian microenvironment for the purpose of improving the oocyte quality. In a recent study, Gallagher et al. [10] suggested that forms of acanthocytosis are associated with either acquired or inherited abnormalities of membrane lipids of red cells.
Deformability of red cells could cause formation of acanthocytes. Clinically, erythrocyte deformability index (DI) is used as an indicator of red blood cells. The DI of erythrocytes changes according to the follicular and ovulatory periods at mid- and late luteal phases [11], and DI is also related to the role of Gonadotropin-Releasing Hormone (GnRH) in inhibiting the synthesis of nitric oxide (NO) [12]. NO acts as a determinant of erythrocyte mechanical behavior and deformability whereas GnRH interacts with the GnRH receptor to releasing the pituitary hormones – follicular stimulating hormone and Luteinizing Hormone (LH).
Currently, it has been widely accepted to use GnRH antagonist protocol at the middle of the controlled ovarian stimulation to inhibit a premature rise in LH [13] for the purpose of preventing eggs being ovulated prior to the egg collection. Indeed, the studies have shown the GnRH antagonist protocol is associated with increased oxidative stress which was confirmed by the levels of malondialdehyde, NO, protein carbonyl, hydroxyl proline, sodium oxide dismutase, reduced glutathione, glutathione peroxidase, adenosine deaminase and xanthine oxidase [14]. NO is one of several intraovarian mediators that have been shown to influence ovarian functions, including follicular development, atresia, ovulation, steroidogenesis, oocyte quality, apoptosis, and luteal function [15]. In addition, NO may positively regulate the expression of angiogenic factors, including Vascular Endothelial Growth Factor (VEGF) and the angiogenesis in the ovaries and other tissues [16].
Reduced follicular vascularity is one of the earliest signs of atresia marked by a smaller vascular network and increased apoptosis in thecal capillaries [17]. Further studies should be conducted as regarding whether the administration of the GnRH antagonist affects the ovarian vascular structures and erythrocytes via nitric oxide pathways or other mechanisms. In addition, the alternative medicine may also benefit ovarian vascular circulation. Recent study showed that oral supplement of Ginger (Zingiber officinale) powder had significant effects on rats in increasing number of antral follicles and expression of VEGF [18]. VEGF expression in human ovaries suggests a role for this growth factor in both cyclic angiogenesis and regulation of vascular permeability, both of which are critical for ovarian folliculogenesis and normal reproductive function [19].
Several other hypotheses of acanthocytes being existed in the follicular fluids would include 1). Primary abnormal microvasculature distribution around follicles; 2). Genetic etiology; 3). Hormonal stimulation used for IVF; 4). Medicines used; 5). Abnormal lipid metabolism that could affect red cell functions; 6) Abnormal angiogenesis. Definitely, existence of acanthocytes can be used as an indicator for the infertility diagnoses, which would help decide clinical treatment plans. Improving circulation of blood supplies to the ovarian follicles would no doubt improve the oocyte quality that eventually increases the pregnancy success rates for the infertility patients. In conclusion, this is the first report that documented the presence of acanthocytes in the follicular fluids of infertile patients, especially the patients diagnosed with endometriosis. The morphology of acanthocytes were further confirmed by the scanning electron microscopy. Discovery of these cells has clinical significance in studying the mechanisms of endometriosis and etiology of unexplained infertility. In addition, the degree of acanthocytes is closely related to the oocyte morphology with significant degenerated oocyte structure in the follicular fluids containing fragmented acanthocytes. These findings provide practical values in decision-making process for taking into consideration of healthy vascular ovarian microenvironment besides the hormonal treatments for the IVF patients. Healthy follicular development is the foundation for obtaining healthy eggs with competence to develop to term [20,21].
Acknowledgement and Funding Information
The author would like to thank Professor Thomas S. Kickler, MD, and Professor Le-Ming Shih, MD, PhD, for their valuable consultations. Both of them are faculty in the Departments of Medicine, Oncology and Pathology at the Johns Hopkins University School of Medicine. The Johns Hopkins School of Medicine Microscope core facility processed the sample for scanning electron microscopy observation. We are grateful for their efficiency in locating the cells for this study. This study has no funding support.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.