Antioxidant Nutraceutical approach to Ewing Sarcoma: Where is the Trap?
Introduction
Nutraceutical antioxidant supplementation in cancer is a controversial and questionable subject, as potential drug-nutrient interactions can affect cancer treatment. Between 13% and 87% of patients with cancer uses antioxidant supplements, often at higher doses compared to those recommended [1,2]. Controversy has been highlighted about the safety of these treatments and the evidences of the efficacy of antioxidant supplement to chemotherapy are indirect and limited [3,4].
The antioxidant nutritional supplements during treatment could alleviate oxidative damage in the healthy tissues, could decrease side effects of treatment and finally could improve the general health and well-being [5-8]. Moreover, tumor response to therapy and patient survival may be improved by the use of antioxidant supplement leading to a better long-term outcome [9,10]. On the other hand, recent literature report that the antioxidant supplementation can affect chemotherapy by reducing its effectiveness. Antioxidants eliminate oxidizing Reactive Oxygen Species (ROS) preventing cellular damage, by affecting antioxidant enzymes by regulating metabolic pathways and providing a adequate supply of non-enzymatic antioxidants [11].
The key enzymatic antioxidant defences are Catalase (CAT), Glutathione Peroxidase (GPx) Superoxide Dismutase (SOD), which constitute the first endogenous defences for the neutralization of ROS. Low amounts of superoxide (O2.-) and hydrogen peroxide (H2O2) are kept by cells, blocking the formation of hydroxyl radicals. Different compounds, eg. Glutathione (GSH), Vitamins A, C and E, Zinc and Selenium represent the non-enzymatic defences [12]. Among the enzymatic antioxidants: SODs convert O2.- to H2O2, catalase reduces H2O2 to water. Moreover, under physiological conditions the reducing power derived from glutathione eliminates H2O2 by molecular oxygen and glutathione peroxidases by the glutathione/glutaredoxin systems. Peroxiredoxin, the Thioredoxin (TRX) are other important mediators of redox signalling in tumor cells [13-19].
When precisely balanced, ROS work as signalling molecules by modulating the activity of the oxidized targets. ROS at low levels promote physiological cells functions by affecting mitogenic proliferation and by acting as second messengers in multiple redox-sensitive signalling reactions [20,21]. The ROS imbalance between pro-oxidant and anti-oxidant processes, either due to excessive ROS production or to decreased scavenging contribute, could lead to an uncontrolled increase of ROS levels that has been considered a common pathway for the onset and progression of many pathologies including cancer, diabetes, neurodegenerative diseases and atherosclerosis. The underlying mechanisms are not yet completely understood. This state of disequilibrium is called ‘oxidative stress’ and can activate all cell pathways involved in cancer progression [12,22]. A “mild” oxidative stress is normally associated with malignant tumour-progression. Cancer cells usually exhibit mitochondrial genomic instability, responsible for metabolic malfunctions and increased production of ROS. Redoxsensitive transcription factors, such as
Nuclear Factor Erythroid 2-Related Factor-2 (NRF2) and the Hypoxia Inducible Factor (HIF-1) once activated, improve antioxidants activities and by increasing cell-survival molecules (Bcl-2 and Akt) [23,24].
The vision of Pediatric Oncologists
Pediatric oncologists have expressed concerns about the supplementation of antioxidants in children with cancer because it could interfere with the anti-tumor activity of conventional therapies [7]. A large proportion of children undergoing treatment for leukaemia, lymphoma and solid tumours suffers from an inadequate growth and a state of malnutrition. The malnutrition in children (6%–50%) depends on the type, stage and location of the tumour [25,26]. For pediatric cancer the debate remains open: should paediatric oncologists provide adequate antioxidant intake, which is essential for normal cellular homeostasis and growth, thereby risking a potential reduction of the effectiveness of anti-cancer therapy, or should not? In this review we evaluate the possible antioxidant supplementations in patients with Ewing Sarcoma (ES). This report will recapitulate available information concerning the pathophysiological role of ROS and antioxidants in cancer and propose a hypothesis concerning a new role of β3-Ars as sensor for the use of antioxidants in Ewing Sarcoma.
Current Data on the use of Nutraceutical Antioxidants
Bioactive nutraceutical compounds with antioxidant ability derived from plants, animals, marine organisms, and microorganisms attracted scientific interest for their beneficial effects on health maintenance. It has been demonstrated that, among natural antioxidant, carotenoids, tocopherols, ascorbates, lipoic acids and polyphenols have strong free radical scavenging activity [27]. Nutraceutical antioxidant supplementation during cancer treatments demonstrated efficacy for many types of malignancies. Interestingly, several anti-tumorigenic drugs derive from natural sources (e.g.: Vincristine, Vinblastine, Paclitaxel, Irinotecan, Topotecan, Etoposide, Daunorubicin, Idarubicin, Actinomycin D) [28,29]. Many bioactive compounds inhibit different pathways involved in tumorigenesis, angiogenesis, and metastasis in pediatric and adult tumors, both in vitro and in vivo by disrupting different cellular enzyme as such as DNA topoisomerase, telomerase. Moreover, antioxidant supplementation interferes with mitotic division, cell-cycle, apoptosis and it eliminates cancer stem cells that are responsible for chemotherapeutic resistance and tumor relapse [30].
Regular consumption of fruits and vegetables reduces the risk of chronic diseases associated with oxidative damage as demonstrated by epidemiological and animal studies [31- 33]. Large-scale epidemiological data reported evidences that treatment with vitamins reduced liver cancer incidence [34]. The supplementation of chlorogenic acid (1µM) suppress glioma growth by affecting M1/M2 macrophage ratio [35]. Interestingly, curcumin (25µM) inhibits the self- glioblastoma stem cell proliferation by inducing intracellular ROS content [36]. In addition, in glioma, combinations of curcumin (0.5µM) and thioridazine (10µM) increase ROS accumulation leading to massive induction apoptosis. In non-small-cell lung cancer Vitamin C treatment exert anti-cancer effects via increasing redox-active labile iron [37] Vitamin E also induce apoptosis in tumour cell lines, increases the efficacy of chemotherapy and reduces drug toxicity [38]. Different vitamin E analogues has been synthesized and screened for their ability to induce human tumour cells to undergo apoptosis [39].
In breast cancer liposome-formulated α-TEA strongly reduced mouse mammary tumour cells growth and lung metastasis in balb/c mice and Vitamin E Succinate (VES) induce DNA synthesis arrest, cellular differentiation and apoptosis leading to cell death [40]. Flavonoids have been reported to have a strong antitumor effect both for antioxidant property and for high cell death induction.
It has been reported that flavonoid apigenin reduces hepatocellular carcinogenesis via decreasing oxidative stress and it exerts anti-tumor effects in various types of cancers, including colorectal cancer, breast cancer, liver cancer, lung cancer, melanoma, prostate cancer and osteosarcoma [41-47]. Apigenin disrupts tumor angiogienesis by revealing similar activity of Axitinib (angiogenesis inhibitor) exerting the same binding activity to the VEGF receptors including VEGFR1 and VEGF R2 [48]. Moreover, this flavone directly inhibits cancer cell proliferation by inducing apoptosis, autophagy and cell cycle arrest. It can reduce oxidative stress, increase hepatic detoxification enzyme efficacy, and act as anti-inflammatory [49,50]. Several studies also reported the synergistic effect of genistein when administered together with other anticancer
drugs by inhibiting MAPK activation and mediating trail induced apoptosis [51]. Despite the great interest of these preclinical data, there are no consistent data regarding the use of flavonoids in human as anticancer therapy. Epidemiologic and clinical trials revealed inconsistent results about the clear dietary recommendations for the intake of flavonoids to support human health [52].
It has been reported that retinoic acid suppress prostate, breast, lung, ovarian, bladder, oral, and skin cancers by causing cell cycle arrest through the inhibition of p27 and Cdk5 and by increasing apoptosis of cancer cells [53]. In pancreatic cancer therapy, combination therapy of gemcitabine with trichostatin A, Epigallocate-3-Gallate (EGCG), capsaicin and Benzyl Isothiocyanate (BITC) have been proven as effective. In addition, Aminoflavone (5-amino-2-(4-amino-3-fluorophenyl)- 6,8-difluoro-7-methylchromen-4-one; AF) is highly selective as inductor of cell death as reported by its ability to kill MCF-7 and MDA-MD-468 breast cancer cells but not non-malignant MCF10A breast epithelial cells. Aminoflavone activates caspase 3 and increases intracellular ROS levels correlating with induction of apoptosis [54,55]. Literature reported that prooxidant toxicity of flavonoids is considered the principal mechanisms by which they inhibit mitochondrial breathing. Cu2+ and Fe2+ which are present in biological systems modulate prooxidant activity of flavonoids, by improving selective cytotoxicity in cancer cells that contain more copper compared with normal cells [56,57].
However, opposites studies keep increasing evidence that the scavenging effect of ROS is in fact deleterious to cancer patients rather than preventing the risk. In another study, N-Acetylcysteine (NAC), a medication that works by increasing the glutathione levels and vitamin E, was shown to accelerate the tumor progression in a B-RAF and K-RAS induced lung cancer in murine models. These antioxidants promote tumour progression by reducing ROS levels and in turn reduce p53 expression levels that could have induced apoptosis leading to the cell death [58]. The role of antioxidants on fighting ROS damage in tumor cells has been implicated in chemoresistance and poor overall survival of cancer patients. Elevated levels of GSH has been indicated as unfavorable factor for the sensitivity to chemotherapeutic agent, as well as high levels of SOD-2 contribute to cancer progression [59,60]. Vitamin A (25,000 IU) supplement leads to an increased risk of lung cancer changing redox homeostasis states in different cancer cell types [61]. In addition, several other investigations have demonstrated that anti-oxidant nutraceuticals accelerate cancer progession by rendering the tumor cells insensitive to elevation of ROS induced by chemotherapic agent [62,63].
Understanding the Controversy
The published literature regarding concurrent chemotherapy or radiation with antioxidants reaches a wide variety of conclusions, some showing improved survival and status and others a reduced survival. Many questions remain open: “Do antioxidants affect the efficacy of anticancer agents by improving toxicity in cancer cells? Do antioxidants protect cancer cells from the effect of chemotherapy?” [31]. The controv ersial role of antioxidants during cancer therapy could depend on the antioxidants dose used: a prophylactic or a therapeutic dose, ie. a low or elevated dose respectively. Prophylactic dose protects healthy cells and tumour cells. On the contrary, a therapeutic dose inhibits the growth of cancer cells but not of healthy cells. On the other hand, higher levels of endogenous antioxidants could adverse oxidative stress induced by chemotherapeutic agents especially in younger patients having impaired capacity to deal with oxidative insult due an increased level of malnutrition status [3,64]. Definitely, dietary antioxidant supplements can be considered as “double-edged sword” in cancer treatment, for their ability either to kill cancer cells or protect them.
The Redox State in Ewing’s Sarcoma Cells
Ewing`s Sarcoma Family Tumors (ESFTs) are round cell tumors that develop in bone and soft tissues of children and young adults [65]. It is a quite common paediatric malignant tumour, with a frequency of 2% of all childhood cancers [66]. Until now combined chemotherapy regimen, radiotherapy and surgery, remains the only strategy to overcome the disease. At diagnosis, approximately 25- 30% of patients with ES have metastatic disease [67]. It presents high incidence of local or distant relapse, up to 40% in metastatic setting. Unfortunately, approximately 20-30% of patients with metastatic or recurrent disease have a poor prognosis even undergoing intensive multi-drug chemoterapy regimen [68]. For patients with localized disease, an intensive multimodal treatment (chemotherapy combined with surgery and/or radiotherapy), achieves a survival rate of approximately 70%. The 10-year survival rate for patients with metastatic disease increased from 16 to 30% after the introduction of multidrug chemotherapy.
Standard chemotherapy includes Adriamicin, Vincristin, Cyclophosphamide and Etoposide. For recurrent disease, only few chemotherapy agents as topotecan and cyclophosphamide, ifosfamide in combination with carboplatin and etoposide, irinotecan and temozolamide showed activity with modest but shortlasting response rate: Patients with metastatic disease often present resistance to chemotherapeutic agents [69].
The origin of ES remain controversial since no precancerous lesions have been described in literature. It is now reported to origin from mesenchymal and neural crest. Over 90% of ES cases present the EWS-FLI1 fusion protein derived from recurrent reciprocal translocations that connect the EWS gene on chromosome 22 with an ETS family gene, either FLI-1 on chromosome 11 or ERG on chromosome 21 [70,71]. EWS-FLI fusion protein is an aberrant protein working as sequence-specific transcription factor leading to neoplastic transformation and tumor progression. Moreover, ES expresses CD99 surface marker, also present in Synovial Sarcoma (SS) and Low-Grade Fibromyxoid Sarcoma (LGFMS). The down regulation of CD99 in human ES cell lines reduced their ability to form tumors and bone metastases in immunodeficient mice and, in vitro, decreased their tumorigenic and metastatic features [72].
Novel therapeutic approaches are needed for metastatic patients resistant to chemotherapy [73]. Preclinical and clinical studies reported drugs targeting EWS-FLI1 fusion protein as small-molecule YK-4-279 that inhibit interaction of EWS-FLI1 and RNA helicase A. Moreover, it has been developed new therapeutic strategy targeting CD99, Vascular Endothelial Growth Factor (VEGF) and its receptor, insulin-like Growth Factor-1 (IGF1) pathways and Mammalian Target of Rapamycin (mTOR), osteoclastic-osteoblastic homeostasis and bone microenvironment, enzymatic pathways (poly ADP- ribose polymerase 1 - PARP1), and GD2 ganglioside pathways [74-78].
Metabolic activity of ES is abnormal with an increased glucose uptake and activation of accelerated glycolysis that provides energy demanded from cancer cells, thus leading to a dysfunction in oxidative phosphorylation activities and redox state imbalance. Therefore, the intracellular redox environment changes rapidly in ES cells. The basis for the growth or the reduction of ES tumour is represented by regulation of ROS production and detoxification balance. Pharmacological approach that decrease cellular antioxidant activity, increasing oxidative stress and inducing of cell death represents one of the most important therapeutic strategy in ES. The antioxidant inhibition strategy has been proposed to enhance the efficacy of chemotherapy as doxorubicin and etoposide which increase generation of ROS and oxidative stress in mitochondria [79]. The majority of conventional drugs used in ES affects ROS balance by increasing the level of ROS over the toxic threshold to kill cancer cells. ES cell lines and patients present different response to the induction of cell death by chemotherapic agents depending on different levels of intracellular antioxidants and the different ability to neutralise ROS [80].
Cellular antioxidants are differentially expressed in ES cell lines. GSH in ES plays an important antioxidant role, therefore, depletion of intracellular GSH enhances the efficacy of ROS-production induced by anticancer agents such as fenretinide leading to a massive cell death [80]. In ES the GSH metabolism pathway altered by upregulation of g-glutamyltranspeptidase enzyme is associated with poor prognosis [81,82]. GSH influences multiple cellular processes, including proliferation, cell differentiation and apoptosis, and its altered homeostasis is involved in the aetiology and progression of different type of cancers and other pathologies. In many tumor cells, elevated GSH levels increase the antioxidant ability and the resistance to oxidative stress while the decrease in the GSH/ GSSG ratio, leads to an increased susceptibility to oxidative stress leading to cancer onset and progression. ES cell lines have high GSH/GSSG ratio that maintain a high basal oxidative stress level which increases the expression of different antioxidants [80].
Recent studies in ES have shown that the Glutathione S-Transferases (GSTs), a family of detoxification enzymes, are direct targets for EWS-FLI1 [83]. GSTs are involved in the development of resistance to cancer chemotherapeutic agents by detoxifying many compounds such as doxorubicin and vincristine [59]. Tumors show high levels of GST expression compared to normal tissues. Over expression of MGST4 (membrane-bound GST4) and MGST1 (membrane-bound GST1) predicts a poor response to chemotherapy as doxorubicin and are associated with poor prognosis [81,83]. MGST1, expressed in the endoplasmic reticulum and outer mitochondrial membrane, protects cells and mitochondria maintaining the homeostasis of glutathione peroxidase function [84]. In Ewing sarcoma cells (SKN-MC) catalase inhibition could represent a potential strategy to increase the sensitivity of ES to chemotherapeutic agents [85]. Various strategies have been studied to increase ROS content in ES cells but none of these proved to be efficacious due to an increased antioxidant response overcoming the activity of chemotherapy. Usually, chemotherapeutic agents increase antioxidants as response to mild levels of ROS, rendering the cells less sensitive to chemotherapy.
New Insight: β3-AR as Prosurvival Factor under Nutraceutical Antioxidant Treatment
Recently, β3 adrenergic receptor (β3-AR) becomes incredibly attractive in cancer biology. Literature reports data about the ability of β3-AR to reduce tumour growth and metastases. Hypoxic induction of β3-AR has been recently reported in tumor microenvironment and overexpression of β3-AR has been associated with cancer growth, recruitment of circulating stromal cell precursors to the tumor sites and enhancement of stem cell traits [86]. The β3-AR antagonist, SR59230A, reduces cell proliferation and induces apoptosis in mouse B16F10 melanoma cells, through a mechanism mediated by inducible isoform of nitric oxide synthase and reduces angiogenesis by inhibiting VEGF secretion. In tumor bearing mice melanoma cell proliferation and tumor vasculature are reduced by intra-tumor injections of SR59230A thus resulting in significant decrease in tumor growth [87].
β3-AR is expressed in bladder, in lymphocytes (at a low level) and in White and Brown Adipose Tissue (WAT, BAT), where its role has been well clarified. β3-AR are involved in adipose tissue morphology and metabolism, administration of selective β3- AR agonist CL- 316,243 increases thermogenesis mediated by Uncoupling Protein 1 (UCP-1) in BAT and lipolysis in WAT [88]. UCPs maintain redox state of the cells in the respiratory chain transport. Over the past decade β3-AR has been proposed as a potential therapeutic target in several pathologies such as cachexia, obesity, diabetes, cardiac disease and in over-active bladder [89], and recent literature reported a new role of β3-AR on cancer progression and dissemination [87].
Interestingly, UCP2 has been shown to control GSH/GSSG in beta pancreatic cells [90]. In particular, as we previously showed in melanoma, β3-AR is expressed on mitochondria driving the activity of UCP-2 and piloting ROS content in the mitochondria [91]. More recently, it has been reported a dual role of β3-AR on antioxidant cell response, it directly inhibits NADPHox activity and induces the expression of catalase. Literature also reported that noradrenaline induces catalytic subunit of Glutamate-Cysteine Ligase Protein (GCLc) thus increasing the intracellular GSH level through stimulation of β3-AR in U-251 MG cells and in mouse astrocytes in primary culture. In this work is reported that β3-AR induces GSH synthesis in glioma cells thus maintaining GSH homeostasis [92,93]. Interestingly, most of the nutraceutical compounds increase β3-AR levels, potentially driving an antioxidant response and cell survival trough activation of ERK or inhibiting the apoptotic process.
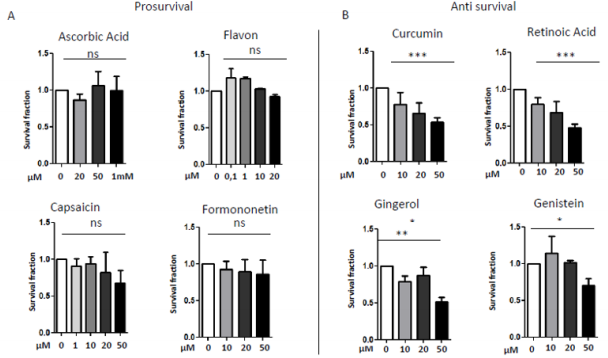
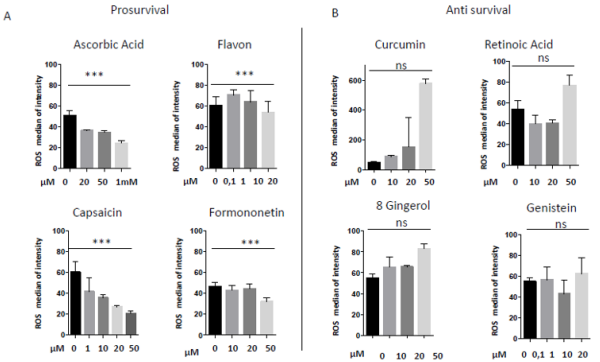
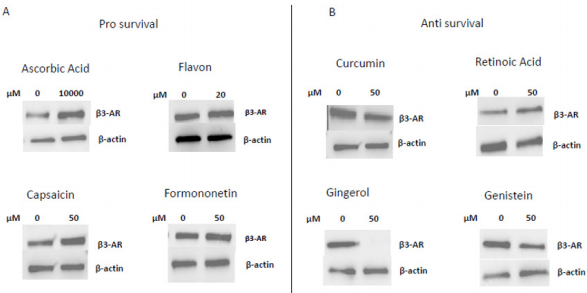
In 1997 was revealed that there is connections between ROS production and UCPs activity in experiments where an inhibitor of UCP1, GDP, increases ∆ψ and ROS production [94]. It has also been reported that ROS production and their levels are correlated with UCPs activation superoxide mediated [95]. Administration of several nutraceutical compounds differentially affect cell survival and apoptosis in ES cells. Specifically, here we report that the ES tumor cells A673 treated with Curcumin, Retinoic Acid, 8-Gingerol and Genistein exhibited reduced viability if compared with A673 cells treated with Capsaicin, Ascorbic Acid, Formononetin and Flavon where the treatment did not affect cell viability (Figures 1A&1B). Moreover, the intracellular expression of mitochondrial ROS remained at low levels in ES cells treated with prosurvival antioxidants and increased in the cells treated with antioxidants that are able to reduce cell viability (Figures 2A&2B). Interestingly, the treatments that that do not affect cell viability up regulated the expression of β3-AR and the treatment that decreased cell viability strongly down regulated β3-AR (Figures 3A&3B).
Figure 1: MTT survival analysis in A673 ES cells treated with different nutraceutical; A) Treatments with Ascorbic Acid (20µM, 5 µM, 1mM), Flavon (100nM, 1µM, 10µM, 20µM), Capsaicin (1µM, 10µM, 20µM, 50µM), Formononetin (10µM, 20µM, 50µM) that did not affect cells viability. B) Treatments with Curcumin (10µM, 20µM, 50µM), Retinoic Acid (10µM, 20µM, 50µM), Gingerol (10µM, 20µM, 50µM) and Genistein (10µM, 20µM, 50µM), that reduced cells viability. NS: not significant. P values for treatments: ∗P <0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 2: Mitochondrial ROS measurement in A673 ES cells treated with different nutraceuticals; A) Decrease of mtROS levels after treatments with Ascorbic Acid (20µM, 50µM, 1mM), Flavon (100nM, 1µM, 10µM, 20µM), Capsaicin (1µM, 10µM, 20µM, 50µM), Formononetin (10µM, 20µM, 50µM). B) Increase of mtROS levels after treatments with Curcumin (10µM, 20µM, 50µM), Retinoic Acid (10µM, 20µM, 50µM), Gingerol (10µM, 20µM, 50µM) and Genistein (10µM, 20µM, 50µM). NS: not significant. P values for treatments: ∗∗∗P < 0.001.
Figure 3: Western Blotting analysis of β3-AR expression after treatment with different nutraceuticals. A) Up-regulation of β3-AR expression after treatment with nutraceuticals that do not affect cells viability: Ascorbic Acid 1mM, Flavon 20µM, Capsaicin 50µM, Formononetin 50µM. B) Down regulation of β3-AR levels after treatment with nutraceuticals that reduce cells viability: Curcumin 50µM, Retinoic Acid 50µM, Gingerol 50µM and Genistein 50µM. β actin is shown as loading control.
Since β3-AR is expressed in the mitochondria, it could drive mitochondrial bioenergetics and the redox state of the cells driving or not cell death under nutraceuticals treatment. Here we identified the β3-AR receptor as the main regulator of the cellular response to oxidative stress in the cells under different micronutrients treatment. It works as sensor for ROS control in ES cells by driving or not cells antioxidant response and cell death. Since β3- AR antagonism lead to massive cell death, inhibiting β3-AR in these cells could dramatically increase the ROS levels by toxic threshold leading to cell death by inhibiting the antioxidant response of the cells.
Since we previously reported that β3-AR antioxidant activity is mediated by UCP-2 protein expression in melanoma, it works as mediator for the elevation of endogenous antioxidants activity of the cells. Here, we report that β3-AR could control the redox state of the cells working as ROS sensor, driving cells to life or to death. It could be possible that this activity is linked to mitochondria bioenergetics function.
Materials and Methods
Cell Cultures
Human Ewing Sarcoma (ES) cells A673 were cultured in 100mm plates in DMEM high glucose medium (4.5 g/L) supplemented with 10% Fetal Bovine Serum (FBS), 5% of L- glutammin, 5% of penicillin and streptomycin (1%) and were mantained at 37°C in a 5% CO2 humidified atmosphere incubator. Cells were usually stored in liquid nitrogen in a freezing solution, containing 95% complete DMEM and 5% Dimethyl Sulfoxide (DMSO) and then plated in petri p100. For defrosting, the vials were rapidly brought to 37°C by immersion in the thermostat bath, then centrifuged to remove the toxic DMSO from the cells, re-suspended in DMEM high glucose FBS 10% and appropriately plated. Sub-confluent cells were detached from plate with trypsin-enzyme after aspirating the medium and after one wash with the Phosphate Buffered Saline (PBS) to eliminate medium and serum residues. Then DMEM high glucose was added and the cell suspension obtained was counted and plated in fresh DMEM high glucose with appropriate dilutions. Hypoxic experiment were performed in hypoxic incubator at 1% of O2 atmosphere.
Cell Treatments
A673 ES cells were plated to reach 70% confluence in complete high glucose DMEM medium. After 24h the medium was removed, the cells were washed in PBS solution, and finally starved overnight with starvation medium, without FBS, in order to promote cell’s entry into a quiescent G0 phase, thereby better evaluating cells responsiveness to exogenous treatments. The consequent morning, cells were treated with a single dose of Apigenin at the concentrations of 10, 20, 50 µM and subsequently left in incubator for 24h, then collected for the experiments. Apigenin was dissolved in dymethylsulphoxide 2,7 mg/ml to obtain a final concentration stock of 10mM, then appropriate dilutions from the stock solution were made for treatments.
MTT Assay
The effect of Apigenin on cell viability was detected by MTT assay. A673 cells were transferred into a 96-wells plate at a density of 10 x 10³ cells/well in 150µl DMEM complete. A673 cells were incubated with various doses of Apigenin (10µM, 20µM, 50µM) for 24 and 48h separetely. A total of 10µl of MTT was added to each well and incubated under darkness for 1h at 37°C. Then culture medium was removed and 150µl of DMSO was added to each well. The intensity of absorbance was detected at 570nm using a dualbeam microplate reader.
Glutathione Fluorometric Assay Kit
For the detection of reduced Glutathione (GSH) the Glutathione Fluorometric Assay Kit (BioVision) was used, following the manufacturer instructions.
MitoSOX and Live/Dead Cells Assays
For the intracellular ROS measurements, A673 cells plated into 24-wells plates at a density of 10 x10⁴ cells in 1ml complete high glucose DMEM medium, were treated with Apigenin as described above. After 3, 6, 24h of treatment, cells were stained with 1µl of MitoSOX reagent at concentration of 2.5µM. After 15 minutes of incubation at room temperature under darkness, cells were washed with PBS, detached with 250µl of accutase buffer, spinned at 1300 rpm for 5 minutes and pellet was resospended in 101µl of live/ dead cells mix (Viobility 488/520 Fixable Dye previously prepared following the protocol). Cells were incubated under darkness for 10 minutes and were washed with 1ml of PBS, spinned at 1300 rpm for 5 minutes and pellet was resospended in 300µl of PBS and then evaluated by flow citometry for MitoSOX-PE reagent.
Western Blot
After homogenization and protein quantifiation, samples were subjeceted to SDS-PAGE and Western Blot analysis. Subsequently PVDF membranes were incubated for 1 hour in slow agitation at room temperature in a blocking solution of non-fat dry milk 2% and Tween PBS 0,05% in order to avoid the formation of unspecific ties. Membranes were then incubated with primary antibody: β3-adrenergic receptor (Abcam), Catalase (Abcam), Superoxide dismutase-2 (Santa Cruz), Txnip (Life Technologies), β-actin (Santa Cruz). The primary antibody was added generally in a concentration of 1:1000 and incubated, in shaking, over- night at 4°C. The next day membranes were washed th ree times with a washing solution containing PBS 1X and Tween 0.1% in order to remove unbound primary antibody in excess. Then, the specific secondary antibody, which was conjugated with Horseradish Peroxidase (HPR), was added, in a dilution of 1:5000 in milk and incubated for 1 hour. Chemiluminescent protein’s revelation was carried out with ECL reagent and developing of blots was carried out by Chemidoc Imaging System (Bio-Rad). To verify the application of equal amounts of protein, the intensity of the corresponding protein bands of interest was normalized on the β-actin bandfor each sample.
Statistical Analysis
in vitro data are presented as means ± Standard Deviation (SD) from at least three experiments. Results were normalized versus control expression levels. Statistical analysis was performed using Graph Pad Prism software (GraphPad, San Diego CA, USA) by OneWay Analysis Variance (ANOVA), followed by Bonferroni post hoc analysis.
Conclusion
in vitro and in vivo data suggest that many nutraceutical antioxidants can enhance or reduce the effects of cytotoxic therapy by differentially altering the intracellular redox state and can promote cells proliferation and malignant progression. Therefore, the use of antioxidants especially in paediatric patients, which are constantly under uncertain nutritional status, remains questionable.
In this review we hypothesize that supplement of determined antioxidants and nutrients can contribute to raise the health status of young patients affected by ES only when nutraceutical supplementation inhibits β3-AR expression. Could antioxidants and β3-AR antagonists be helpful in Ewing Sarcoma? SR5920A could increase oxidative stress clarifying the way for the use of antioxidants to kill cancer cells and maintain the wellness of normal cells where this receptor is expressed at a lower level. Moreover, we showed that SR59230A strongly reduces cancer cell viability, demonstrating that blocking of β3-AR function could represent a novel therapeutic strategy for ES through its ability to reduce antioxidant activity [96].
Acknowledgment
Thanks to Margherita Nardi and Marina Vignoli for kindly revised the manuscript.
For more Articles: https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.