Selective Vulnerability of Nerve Demyelination in Chronic Acquired Multifocal Polyneuropathy
Introduction
The pathological features of peripheral nerve myelin lesion
are known as diffuse and focal demyelination. As a hallmark of
peripheral nerve focal demyelination, conduction block (CB) has
been found in a series of both acquired and hereditary demyelinating
neuropathies [1-4]. Multifocal motor neuropathy (MMN) and
Lewis-Sumner syndrome (LSS) are the most representative
chronic acquired focal neuropathies, while many of the classic
chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) also presents with CB [5]. Distinct from common peripheral
neuropathies, the motor impairments in these focal neuropathies
are clinically uneven, i.e., non-length dependent and asymmetric
[6,7]. Their limb onset selectivity is well-known [8], however, not
efficient in reciprocal differentiation. Different nerve involvement
among demyelinating polyneuropathies was proposed by the
morphological study, which suggested the selective vulnerability
of nerves [9]. The selectivity was observed in other neuropathies
[10,11], but hasn’t been fully studied in focal demyelination by
electrophysiology methods. Thus, in this study, by using of a bunch
of well-established electrophysiological indicators, e.g., distal
motor latency (DML), terminal latency index (TLI), F-wave latency
and nerve conduction velocity (NCV) as well as CB, we aimed
to investigate the selective vulnerability and the demyelinating
pattern of nerves and segments in patients with MMN, LSS and
CIDP with CB (CIDP-CB).
Materials and Methods
Subjects
This was a retrospective cross-section study performed under the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Renji Hospital Shanghai Jiaotong University School of Medicine. Written informed consent was obtained from the subjects for publication of this paper. Fifteen LSS subjects, 24 MMN subjects and 17 CIDP-CB subjects admitted at Renji Hospital Shanghai Jiaotong University School of Medicine from 2013 to 2017 were included in the study. A total of 107 nerves of LSS subjects, 176 nerves of MMN subjects, and 110 nerves of CIDP-CB subjects were analyzed. The LSS and CIDP diagnoses were based on the 2010 European Federation of Neurological Societies (EFNS)/Peripheral Nerve Society (PNS) guideline on CIDP [12], and the MMN diagnose was based on the 2010 EFNS/PNS guideline on MMN [13]. The exclusion criteria were peripheral neuropathy family history; alcohol abuse; toxic and neurotoxic exposure; tumor; metabolic disorders including pathoglycemia (diabetic mellitus and impaired glucose tolerance); paraproteinemia (monoclonal gamma-globin related polyneuropathy).
Clinical Profiles and Examinations
General information of gender, age and height were recorded. Detailed clinical history was investigated to collect disease duration and onset and determine neurological manifestations of weakness, numbness and atrophy. Meticulous physical examinations were performed to determine tendon reflex as well as other positive neurological signs. Blood routine test, liver and kidney function, blood electrolytes, thyroid function, vitamin B12, folic acid and blood glucose were checked to meet the inclusion and exclusion criteria. Gangliosidosis antibodies examination were semi-quantitative by blot and was fulfilled by KingMed Diagnostics (Guangdong, China). A panel of various kinds of anti-Gangliosidosis (GM1, GM2, GM3, GD1a, GD1b, GQ1b, GD1b) was used and positive result was determined by visible band in GM1 area. Blood immunofixation electrophoresis was tested for subjects to meet the exclusion terms. Lumbar puncture was performed under the informed consent of subjects and cerebrospinal fluid (CSF) was quantitatively analyzed to determine albumin protein concentration.
Nerve Conduction
All subjects received nerve conduction test by Keypoint. net (Natus, CA, USA) in a quiet room with temperature >20℃. Surface electrodes were used to record wave form on fully relaxed muscle after stimulation. Median, ulnar, radial, peroneal, peroneal superficial, tibial and sural nerve were selected for evaluation (sensory nerve data were not shown). Multiple sites were stimulated for every nerve to obtain sufficient data and calculate velocities. For median nerve, recording was placed on abductor pollicis brevis (APB), and stimulation was conducted on wrist and elbow. For ulnar nerve recording was placed on abductor digiti quinti (ADQ), and stimulation was conducted on wrist, below elbow, above elbow and Erb’s point. For radial nerve recording was placed on extensor indicis proprius (EIP), and stimulation was conducted on forearm, below elbow and upper arm. For peroneal nerve, recording was placed on extensor digitorum brevis (EDB), and stimulation was conducted on ankle, below fibular head and above fibular head. For tibial nerve, recording was placed on abductor hallucis (AH), and stimulation was conducted on ankle and popliteal fossa. Normal values and ranges of our lab were adopted. Negative peak amplitude, curve area and duration were recorded to determine CB and temporal dispersion (TD). A definite CB was defined as area reduction on proximal vs. distal stimulation of at least 50%, distal compound muscle action potential (CMAP) must be >20% of the lower limit of normal and >1 mV, and increase of proximal to distal CMAP duration must be ≤30%.13 Nerve variant especially Martin-Gruber anastomosis was carefully excluded by additional stimulation. Temporal dispersion was defined as >30% duration increase between the proximal and distal CMAP12.
Take-off latency and distance were recorded and NCV was thus calculated. Distal distance (dD), DML, and NCV was used to calculate TLI by the following formula: TLI=(dD/DML)/NCV. F-wave was performed during a bout of stimulation recorded on distal muscle and the first F-wave latency (F) was recorded. DML, proximal motor latency (PML) which is the latency recorded from the second stimulation site from distal, and F were used to calculate modified F-wave ratio (MFR) by the formula:
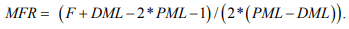
Statistical Analysis
Median, ulnar, radial, peroneal and tibial nerves were selected
for studying CB and TD distributions. Median, ulnar, peroneal
and tibial nerves were selected for comparing other conduction
parameters. Normal distribution data were described as mean
 ± standard deviation (SD), and skewed distribution data
were described as median (25% percentile – 75% percentile). Particularly, data of the same type were kept the same description
in the tables for comparability. Pearson and Fisher chi-square tests
were used to analyze the proportion and composition of different
groups. Intragroup composition was analyzed by binomial exact
test. One-way analysis of variance (ANOVA) and group t-test were
used for data with normal distribution and standard homogeneity
of variance, otherwise Kruskal Wallis test and Wilcoxon Ranksum
test were chosen instead. All statistics were performed by Stata 15.1
(StataCorp, TX, USA) and p<0.05 indicated significant differences.
± standard deviation (SD), and skewed distribution data
were described as median (25% percentile – 75% percentile). Particularly, data of the same type were kept the same description
in the tables for comparability. Pearson and Fisher chi-square tests
were used to analyze the proportion and composition of different
groups. Intragroup composition was analyzed by binomial exact
test. One-way analysis of variance (ANOVA) and group t-test were
used for data with normal distribution and standard homogeneity
of variance, otherwise Kruskal Wallis test and Wilcoxon Ranksum
test were chosen instead. All statistics were performed by Stata 15.1
(StataCorp, TX, USA) and p<0.05 indicated significant differences.
Results
General Information of Subjects
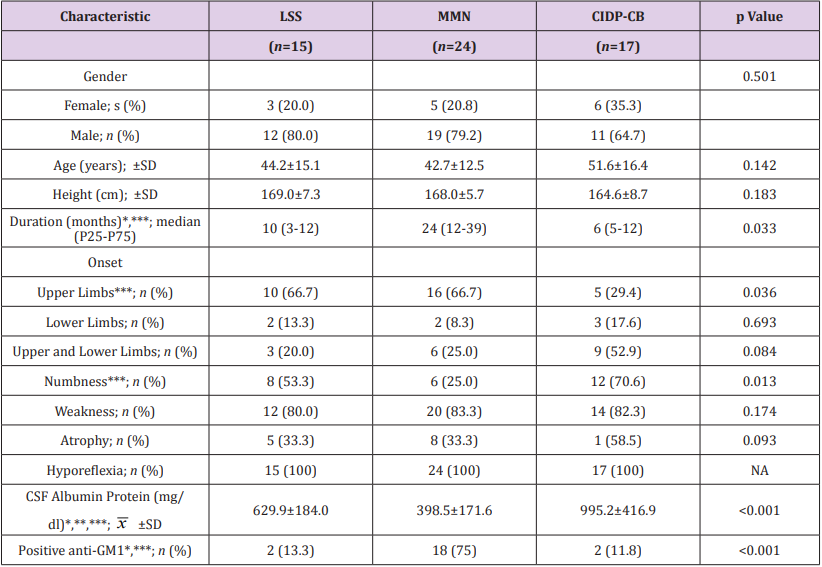
Gender, age and height were commensurate among different groups (p>0.05). Intragroup gender composition was significant in LSS group (p=0.035) and in MMN group (p=0.007), but not significant in CIDP-CB group (p=0.332). MMN group showed a longer disease duration compared to other groups (p<0.05). Pure upper limb onset and sensory involvement were less probable in CIDP-CB group compared to MMN group (p<0.05). There was no significant difference found in other clinical symptoms or signs among groups (p>0.05). CSF albumin protein showed a gradient elevation from MMN to LSS to CIDP-CB (p<0.001). Anti-GM1 was most likely to be detected in MMN group (p<0.001). General and clinical comparisons are shown in Table 1.
Table 1: General and clinical profiles of LSS, MMN and CIDP-CB subjects.
Note:
CIDP-CB, Chronic inflammatory demyelinating polyneuropathy with conduction block; CSF, Cerebrospinal Fluid; GM1, Monosialotetrahexosyl ganglioside; LSS, Lewis-Sumner syndrome; MMN, Multifocal motor neuropathy; NA, Not applicable.
*p<0.05 between LSS subjects and MMN subjects; ** p<0.05 between LSS subjects and CIDP-CB subjects; *** p<0.05 between MMN subjects and CIDP-CB subjects.
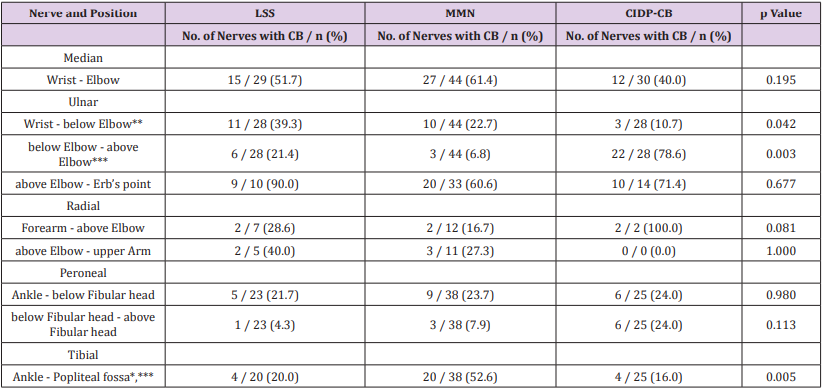
CB And TD Distribution of Nerves
CB was more likely to occur around elbow (p<0.01) while less likely to be found between wrist and elbow in CIDP-CB (p<0.05). CB was found in more than half of tibial nerve examined in MMN and it was significant (p<0.01). No significant difference of CB occurrence was found in other sites. TD was found in 7 of 107 nerves in LSS, 12 of 176 nerves in MMN and 8 of 110 nerves in CIDP-CB. The comparison was statistically insignificant (p=0.977). CB distribution is shown in Table 2.
Table 2: Nerve distribution of CB.
Note:
CB, Conduction Block; CIDP-CB, Chronic inflammatory demyelinating polyneuropathy with conduction block; LSS, Lewis-Sumner syndrome; MMN, Multifocal motor neuropathy.
* p<0.05 between LSS nerves and MMN nerves; ** p<0.05 between LSS nerves and CIDP-CB nerves; *** p<0.05 between MMN nerves and CIDP-CB nerves.
Conduction Indicators of Nerves
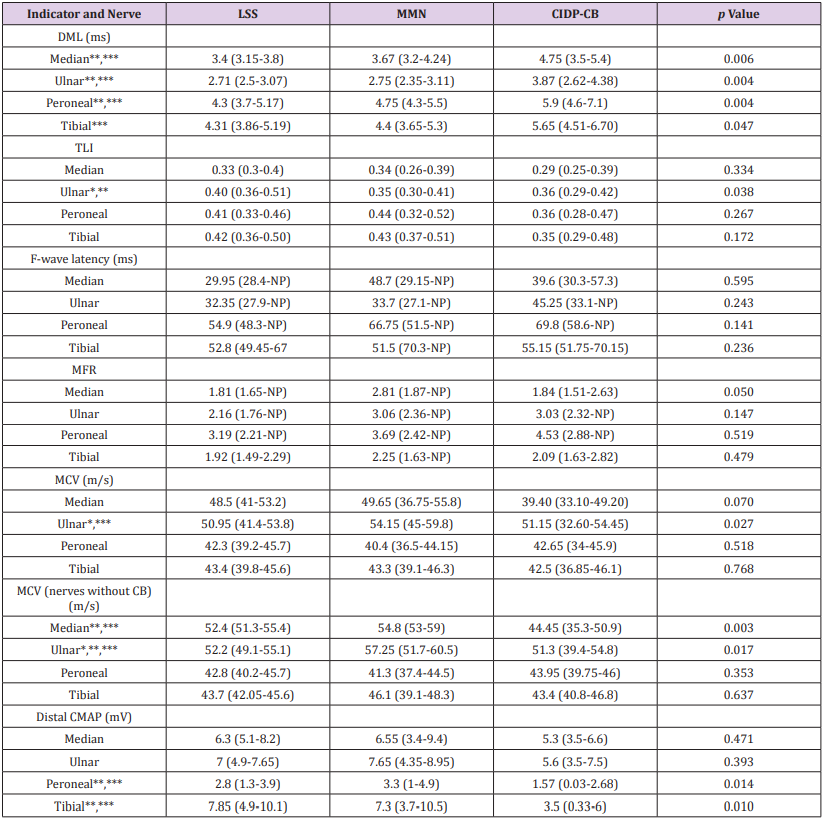
DML was significantly prolonged in CIDP-CB in all nerves (p<0.05), while only ulnar nerve TLI prolonging was observed in LSS (p<0.05). No F-wave latency or MFR difference was found in any nerve. Ulnar nerve NCV differences were found among groups (p<0.05). When nerves with CB were excluded, decrease of upper limb nerve NCV was statically significant (p<0.05). Lower limb CMAP was lower in CIDP-CB (p=0.01). Electrophysiological findings of the indicators are shown in Table 3.
Table 3: Electrophysiological findings of peripheral nerve demyelination among LSS, MMN and CIDP-CB nerves.
Note:
CB, Conduction block; CMAP, Compound muscle action potential; CIDP-CB, Chronic inflammatory demyelinating polyneuropathy with conduction block; DML, Distal motor latency; LSS, Lewis-Sumner syndrome; MCV, Motor conduction velocity; MFR, Modified F-wave ratio; MMN, Multifocal motor neuropathy; NP, No potential elicited; TLI, Terminal latency index. Results are show as median (P25-P75). No. of nerves: LSS, Median=29, Ulnar=28, Peroneal=23, Tibial=20; MMN, Median=44, Ulnar=44, Peroneal=38, Tibial=38; LSS, Median=30, Ulnar=28, Peroneal=25, Tibial=25.
* p<0.05 between LSS nerves and MMN nerves; ** p<0.05 between LSS nerves and CIDP-CB nerves; *** p<0.05 between MMN nerves and CIDP-CB nerves.
Discussion
MMN and LSS were characterized by male predominant, upper limb onset and different sensory involvement [14-17]. In this study we also found a male predominant composition in the MMN group and LSS group and it was different in the CIDP-CB group. High upper limb onset proportion and rare sensory involvement in MMN were also proved and could help in differentiating CIDP-CB. In addition, we found MMN presented slowly progressive long duration, low CSF protein and frequent positive anti-GM1, while LSS and CIDP presented progressive duration, moderate-high CSF protein and rare positive anti-GM1. Clinical findings were confirmatory and consistent with previous study [18,19]. Using electrophysiological indicators, we presented a horizontal comparison of LSS, MMN and CIDP-CB nerves. Selective vulnerability of nerve is particularly interesting and controversial in focal neuropathy. Ulnar nerve has been suggested useful in diagnosing acute demyelinating neuropathy [20], and tibial nerve was hypothesized to be especially involved in MMN [21], while other study found no difference in CB distribution [22]. In this study, noting that wrist to elbow segment of ulnar nerve conduction was least likely to be blocked in CIDPCB compared to LSS, while the CB likelihood of segment around ulnar was vice versa in CIDP-CB compared to MMN. Another CB distribution characteristic we found was the frequent tibial nerve CB in MMN. Although upper limb is more frequently affected in MMN, superimposing of tibial nerve focal demyelination implies inclination of MMN diagnose. These findings suggest that ulnar and tibial CB may be critical in differentiation, and to our knowledge hasn’t been reported in chronic focal demyelinating diseases.
The mechanism of nerve selectivity of chronic focal demyelinating diseases hasn’t been fully investigated. Recently, a common etiology discovered in demyelinating diseases with CB was the detection of autoantibodies, e.g., anti-neurofascin 140/186, anti-neurofascin 155 [23,24]. As a target, the neurofascin expression in node and paranode sites determines the impairment pattern of conduction failure and is associated with CB [25]. A reasonably hypothesis is that the uneven distribution of nerve expression of neurofascin may contribute to the nerve selectivity.
Distal vulnerability was suggested in typical CIDP previously [26,27], while, CIDP-CB, as a subtype, has rarely been analyzed and compared solely. Although prolonged DML in all CIDP-CB nerves was in accordance with the distal predominant pattern, the similar values of TLI, a comparison of distal and middle segments [28], of most nerves among groups implied that the distal impairments might commensurate with middle segment when the existence of CB was taken into consideration. The CB also masked the different diffuse demyelinating of median nerve as only ulnar nerve MCV was lower in CIDP-CB. When the nerves with CB were excluded, upper limb MCV decrease in CIDP-CB emerged. In the conduction study, we proved that CIDP-CB showed many common features with typical CIDP comparing to MMN and LSS and this highlighted its diffused demyelination feature in median and ulnar nerves.
Conclusion
We reported the difference of selective segmental vulnerability
of ulnar and tibial nerves in LSS, MMN and CIDP-CB, and proved more
diffuse demyelinating features in CIDP-CB which distinguished it
from LSS and MMN. The different nerve demyelination distribution
and pattern could help in differentiation. This study was limited by
insufficient proximal and distal conduction data and small sample.
A prospective cohort and immunological mechanism study are
anticipated.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.