Activated Protein C (APC) Promotes A Healing Phenotype in Cultured Murine Tenocytes Via Protease- Activated Receptor (PAR)-2, but not PAR-1
Background
Tendon is the connective tissue that transmits the force from
muscle to bone to facilitate joint movement. Healthy tendon is
comprised of fibroblast-like tenocytes between parallel collagen
fibres. Injury to a tendon triggers an ordered triphasic healing
response: (i) Inflammation, (ii) Repair and (iii) Remodeling [1].
Hindrance to these sequential stages can halt the healing cascade,
leading to tendinopathy.
‘Tendinopathy’ is a non-specific term used to describe pathology in, and/or pain arising from a tendon. Indications of tendinopathy include collagen disorganization, increased cellularity and a poor tendency to heal [2,3]. Tendon injuries cause considerable morbidity in the general adult population [4]. The ideal treatment for tendinopathy is yet to be elucidated and should be focused on elucidating the key functional pathways implicated in the disease [5]. Activated protein C (APC) is an endogenous serine protease of physiological importance due to its potent anti-coagulant, anti-inflammatory and cytoprotective properties [6]. Protein C is mostly produced by the liver and is secreted to the blood where it is activated to APC when bound to the thrombin-thrombomodulin complex. Endothelial protein C receptor (EPCR) can enhance this activation. Once activated, APC exerts either its anti-coagulant activity, or while still bound to EPCR it can cleave protease activated receptors (PARs) to elicit cytoprotective effects via numerous signaling pathways, including inhibition of the nuclear factor (NF)- κB, and activation of the mitogen-activated protein (MAP) kinase and glycogen synthase kinase (GSK)-β3 pathways [7]. PAR-1 and PAR-2 have been found to be vital to cell functions in various body systems including musculoskeletal system [8,9], the nervous system [10], cardiovascular system [11], respiratory system [12,13], as well as the integumentary system [14-24].
In a previous study, APC has been shown to stimulate a healing phenotype in sheep tenocytes via the EPCR [25]. APC increased tenocyte proliferation, matrix metalloproteinase (MMP)-2 activity and collagen type I deposition in a dose and time dependent manner [25]. Additionally, the MAP kinase pathway was proposed to be involved; APC dose-dependently stimulated phosphorylated (P)- extracellular signal-regulated kinase (ERK)-2 and inhibited P-p38 [25]. Whilst APC has been shown to exert some of these effects on tenocytes via EPCR, whether and how PARs are involved remains to be elucidated. Understanding the molecular mechanisms of APC is crucial in maximizing its therapeutic potential in tendinopathy.
Methods
Aim
The aim of this study was to determine whether APC stimulates murine tenocyte healing and if so, to assess the involvement of the receptors and underlying mechanisms in vitro.
Cell Isolation, Culture and Treatment
Three weeks old female wild type (WT), PAR-1 knock out (KO) or PAR-2 KO mice (all are with a C57 background) were bred and obtained from Kearns Facility, Kolling Institute, University of Sydney. 6 mice were used for each gene knockout with a total of 18 mice used. Mice were euthanized by a trained, individually; separate from animal room, in a visible chamber, with 100% carbon dioxide with a fill rate of 70% of the chamber volume per minute. Mice were observed for cessation of respiration within 2 minutes and carbon dioxide flow continued for another 1 minute thereafter. After euthanized, mouse-tail skin was physical peeled back; tendon stripped off and cut into an amorphous mass of small pieces. Tenocytes were extracted from the tail tendon using a 0.2 % Type 1 collagenase digestion medium and then cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % foetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin. The unused mouse tissues were cremated and discarded. Confirmation of complete deletion of PAR-1 and PAR-2 at the gene level was further achieved by reverse transcription (RT)-PCR. After confluency, cells were trypsinized, reseeded into individual 24-well culture plates, and grew. When approximately 90 % confluency was reached, cells were switched to serum free DMEM overnight, then changed to fresh serum free DMEN and treated with recombinant human APC (Eli Lilly, Indianapolis, Indiana USA).
After treatment, culture supernatants were collected for zymography and cells were lysed by NET lysis buffer (100 mM NaCl, 1 mM EDTA, 20 mM Tris, 0.5 % Triton X100) supplemented with protease and phosphatase inhibitors (Roche, Sydney NSW Australia) for western blot. Cells from passages 1 to 4 were used in experiments. Royal North Shore Hospital Animal Ethics Committee approved usage of mouse tissues. All experiments were performed three times.
Gelatin Zymography
MMP-2 and MMP-9 protein secretion and activation in the cell culture supernatants were detected by gelatin zymography under non-reducing conditions, as described previously [26]. In brief, the proteins were separated by electrophoresis under non reducing conditions with gelatin retained in the gel. After electrophoresis, the gel was renatured with Triton® X-100, and subsequently developed in an appropriate activation buffer. During this development, the concentrated, renatured MMPs in the gel digested the substrate. After incubation, the gel was stained with Coomassie® Blue, and the MMPs were detected as clear bands against a blue background of undegraded substrate. The clear bands in the gel were then quantified by densitometry.
Western Blotting
The expression and activation of ERK1/2, AKT and GSK-β3 by tenocytes were investigated by Western blotting as described previous [25] β-actin was included to assess equal loading.
MTT Assay
Tenocyte proliferation was assessed by the 3- (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cells were counted and seeded in a 96-well plate with 200 μl of 10 % FBS in DMEM. After overnight attachment, the medium was replaced with fresh 2 % FCS DMEM and APC treatments were applied. Cells were incubated at 37 °C for a total of 72 hrs. Three hrs prior to termination of the experiment, 10 μl of 2 mg/mL MTT solution was added to each well. After 3 hrs incubation, the medium was removed and replaced with 100 μl of dimethyl sulfoxide. The colour change resulting from the solubilisation of formazan crystals was quantified using a microplate spectrophotometer (BioRad) operating at 570 nm. A baseline reading was also taken at 630 nm to minimize background interference.
Scratch Wound Assay
Tenocyte migration was examined via a scratch assay. Cells were counted and seeded into 24-well plates and grown to 70% confluency. One vertical line was scratched down the center of each well using the point of a sterile 1 mL pipette tip, creating a cellfree “wound” area approximately 2 mm in width. To standardize the position of the wound when photographing, small indents were made in the well using a sterile 31G needle. Cells were washed twice with 1 mL of media to flush away any suspended cells. Cells were then starved in 2 % FBS DMEM and photos immediately taken. To distinguish the contribution of proliferation to the migration of tenocytes into the “wound” area, cells were pre-treated with mitomycin C (10 μg/mL, Sigma, Aldrich) 2 hrs before “wounding” to inhibit proliferation. Cells were then treated with 1 μg/mL APC and photos were taken again after 24 hr. Cell migration was determined by calculating the fold change of cells that migrated into wound areas in 24 hours by:
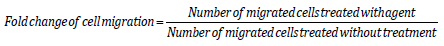
mRNA Isolation and Quantitative Real Time PCR
mRNA was isolated using RNAzol RT Isolation Reagent (Molecular Research Center, Cincinnati, OH USA). Primers for EPCR, PAR-1 and PAR-2 were designed and checked for specificity using the National Center for Biotechnology Information BLAST search tool [27]. The murine primer sequences for EPCR (NM_011171.2: 183bp) were 5ʹ′-ATCTGACCCAGTTCGAAAGC-3ʹ′ (forward) and 5ʹ′- GGCCGGAAACTTACAAAAGC-3ʹ′ (reverse); PAR-1 (NM_010169.3; 199bp) were 5ʹ′- ACTTCACTTGCGTGGTCATT -3ʹ′ (forward) and 5ʹ′- GAAACGATCAACGGCACAAG-3ʹ′ (reverse); PAR-2 (NM_007974.4; 164 bp) were 5ʹ′-CCTTACTGCATCTGCCTACG-3ʹ′ (forward) and 5ʹ′- AATGCACTACGAGCAGAAGG -3ʹ′ (reverse). RT Quantitative PCR was performed to determine the amounts of EPCR, PAR-1 and PAR- 2 gene expression in tenocytes from WT, PAR-1 KO and PAR-2 KO mice (The Rotor-Gene 6000 Real-Time PCR machine, Corbett Life Science, Mortlake Australia). Data were analysed using the relative quantification method, and results expressed as fold change (ΔRn) relative to wild type (WT) untreated samples. β-Actin housekeeping gene expression was used to normalize mRNA levels of PAR-1, PAR- 2 and EPCR.
Statistical Analysis
All data was expressed as mean ± standard deviation (SD). Results were analysed using one-way analysis of variance (ANOVA), in combination with and followed by Tukey post-hoc test. GraphPad Prism software was used for statistical computations. A p-value < 0.05 was considered statistically significant.
Results
Gene Expression of EPCR, PAR-1 or PAR-2 in WT, PAR-1 and PAR-2 KO Cells
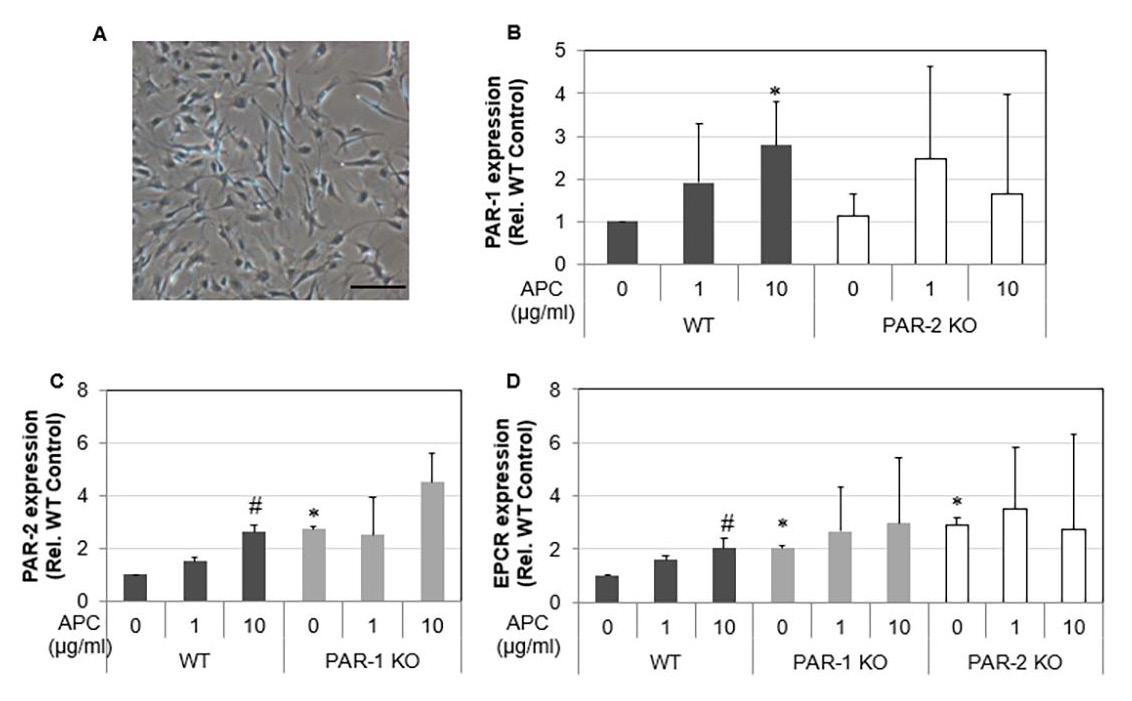
A homogenous cell population was obtained and morphologically identified as fibroblast-like cells. Spindle-shaped contours were observed using inverted phase-contrast microscopy (Figure 1A). Tenocytes from 3 w/o mice demonstrated that, as expected, PAR-1 KO cells had no expression of PAR-1 and PAR-2 KO cells had no expression of PAR-2 (data not shown). Interestingly, expression of PAR-1 by PAR-2 KO cells did not change whereas PAR-2 expression was increased 2.7 folds by PAR-1 KO cells. EPCR expression was stimulated in both PAR-1 KO and PAR-2 KO cells (Figures 1B-1D). In WT cells, EPCR, PAR-1 and PAR-2 expression showed a dose-dependent response to APC treatment. There were 1.6 and 2-fold increases (p<0.05) in EPCR expression in response to APC at 1 and 10 μg/mL, respectively (Figure 1D); a 2.7-fold increase in PAR-1 expression and a 2.7-fold increase (p<0.05) in PAR-2 expression at 10 μg/mL APC by WT tenocytes (Fig. 1B&C). However, the expression of PAR-2 and EPCR by PAR-1 KO tenocytes or the expression of PAR-1 and EPCR by PAR-2 KO tenocytes did not display a statistically significant response to APC treatment (Figures 1B-1D). This data indicates that APC can regulate its receptor expression, while knockout PAR-1 or PAR-2 abolishes this effect of APC.
Figure 1: The gene expression of EPCR, PAR-1 and PAR-2 in WT, PAR-1 KO and PAR-2 KO tenocytes in response to APC.
Note: Tenocytes at passage 1 from 3 w/o WT, PAR-1 KO and PAR-2 KO mice were treated with APC (1, 10 μg/ml) for 24 hrs.
A) Micrograph of tenocytes growing from collagenase digestion tendon at passage 1. Scale bar: 100 μm.
B) Gene expression of PAR-1 in WT and PAR-2 KO tenocytes.
C) Gene Expression of PAR-2 in WT and PAR-1 KO tenocytes.
D) Gene expression of EPCR in WT, PAR-1 KO and PAR-2 KO tenocytes. Quantitative RT-PCR of EPCR, PAR-1 and PAR-2
expression in tenocytes normalized to β-actin. Bars show mean ± SD (n=3). *p< 0.05 vs WT Control and #p< 0.05 vs their own
controls, one-way ANOVA calculated using Tukey post-hoc analysis.
Proliferation and Migration of Tenocytes
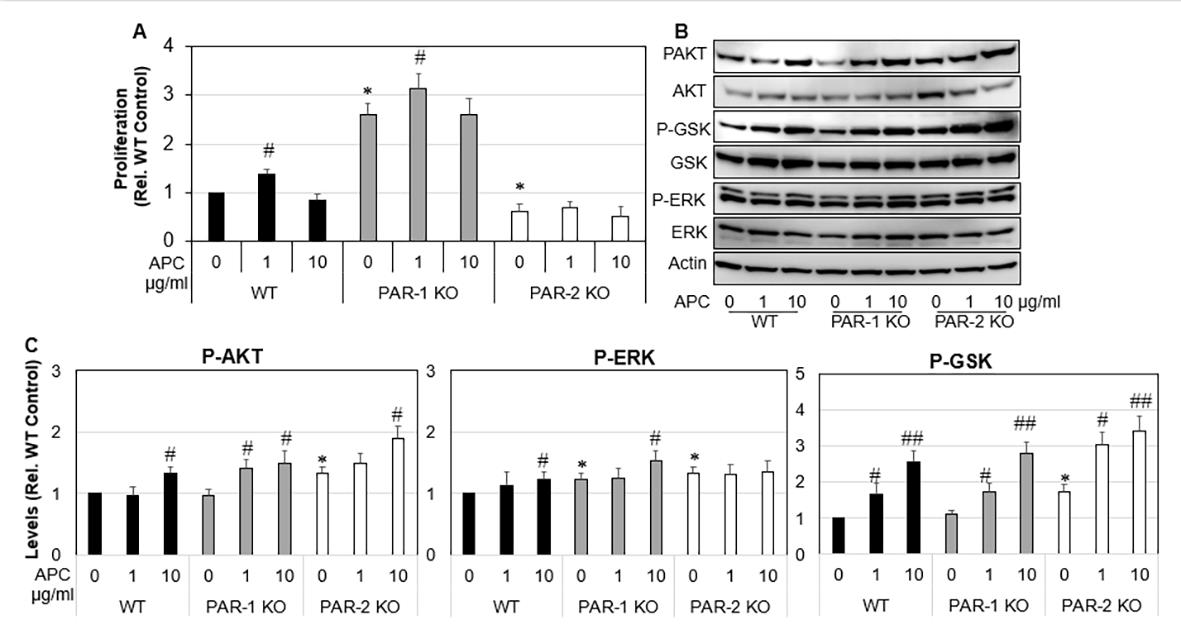
Figure 2: Proliferation and MAP Kinase expression of WT, PAR-1 KO and PAR-2 KO tenocytes in response to APC.
Note: WT, PAR-1 KO and PAR-2 KO tenocytes were treated with APC (1, 10 μg/ml) for either 24 hrs or 72 hrs.
A) Tenocyte proliferation assessed 72 hrs after APC treatment by MTT assay.
B) Expression and activation of ERK, AKT and GSK-β3 24hrs after APC treatment detected by Western blot.
C) Data are semi-quantitation by Image J and depicted in the graph as fold change relative to control. Results shown are mean
± SD (n=3) *p< 0.05 vs WT Control and #p< 0.05 vs their own controls (no treatment), one-way ANOVA calculated using Tukey
post-hoc analysis. ##P<0.01.
Cell proliferation and migration are vital for tendon healing. Under basal conditions, proliferation of PAR-1 KO tenocytes was increased by 3.3-fold compared WT tenocytes (Figure 2A), p<0.0001). In contrast, PAR-2 KO tenocytes showed a 0.6-fold decrease in proliferation when compared to WT control. APC promoted proliferation of WT tenocytes by ~1.3-fold (p<0.05), PAR- 1 KO tenocyte by 1.2-fold (p<0.05) (Figure 2A) at 1 μg/ml when compared to their own controls (Figure 2A). APC had no significant effect on the proliferation of PAR-2 KO tenocytes (Figure 2A). These results suggest that APC dose-dependently promotes tenocyte proliferation, similar to other cells showed previously [28], and this stimulating effect is likely via PAR-2. To investigate the underlying mechanisms, western blot was performed to examine the activation of ERK, AKT and GSK-β3, three intracellular molecules that associated with cell proliferation/survival [7,29-32]. Compared to WT, activated forms of ERK, AKT and GSK-β3 were significantly higher in PAR-2 KO cells (Figures 2B & 2C). APC stimulated the activation of AKT and GSK-β3 in all primary cells, and the activation of ERK in WT and PAR-1 KO cells (Figures 2B & 2C).
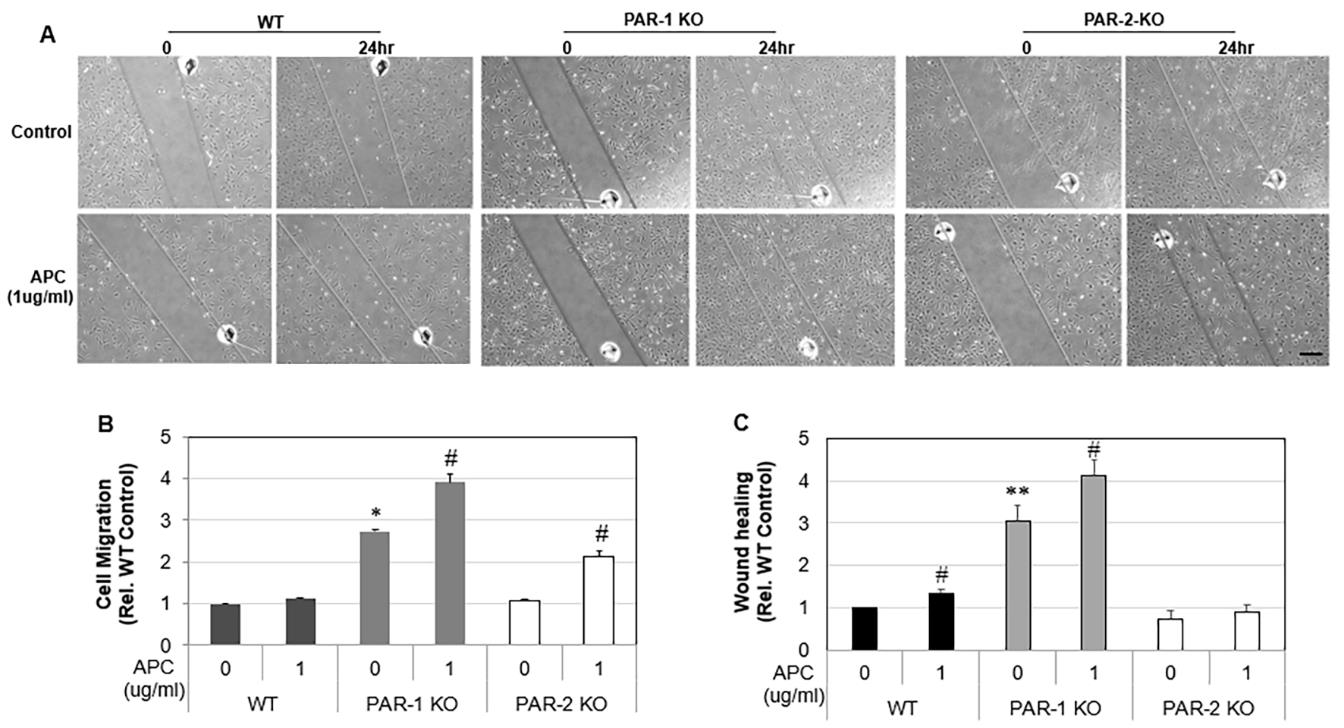
Similar to proliferation, unstimulated PAR-1 KO tenocytes showed a 2.7-fold (p<0.0001) increase in cell migration compared to WT cells, whereas PAR-2 KO tenocytes did not differ from WT control. APC (1 μg /mL) had no effect on WT tenocyte migration (Figures 3A & 3B), but stimulated PAR-1 KO tenocyte migration by 1.4-fold (p<0.0001) and PAR-2 KO tenocytes migration by 2.1- fold (p< 0.001), when compared to their own controls (Figures 3A & 3B). Wound healing is a combined effect of cell migration and proliferation. As expected, wounds created in PAR-1 KO cell monolayers healed 3-fold faster than WT cells (Figure 3C), whereas wounds on PAR-2 KO cell monolayers healed at a similar rate to that by WT control. APC at 1 μg/ml promoted ~1.3-fold increase in wound healing by PAR-1 KO and WT by not PAR-2 KO cells (Figure 3C).
Figure 3: Migration and wound healing of WT, PAR-1 KO and PAR-2 KO tenocytes in response to APC.
Note: WT, PAR-1 KO and PAR-2 KO Tenocytes were treated with APC for 24 hrs.
A) Representative images of migration assay.
B) Tenocyte migration assessed 24 hrs after APC treatment (1 μg/ml) by a scratch assay.
C) Tenocyte wound healing assessed 24 hrs after APC treatment (1 μg/ml) by a scratch assay. Results are expressed as mean
± SD (n=2). Scale bar: 250 μm. *p< 0.05 vs WT Control and #p< 0.05 vs their own controls, one-way ANOVA calculated using
Tukey post-hoc analysis. **p<0.01.
MMP-2 and MMP-9 Expression by Tenocytes
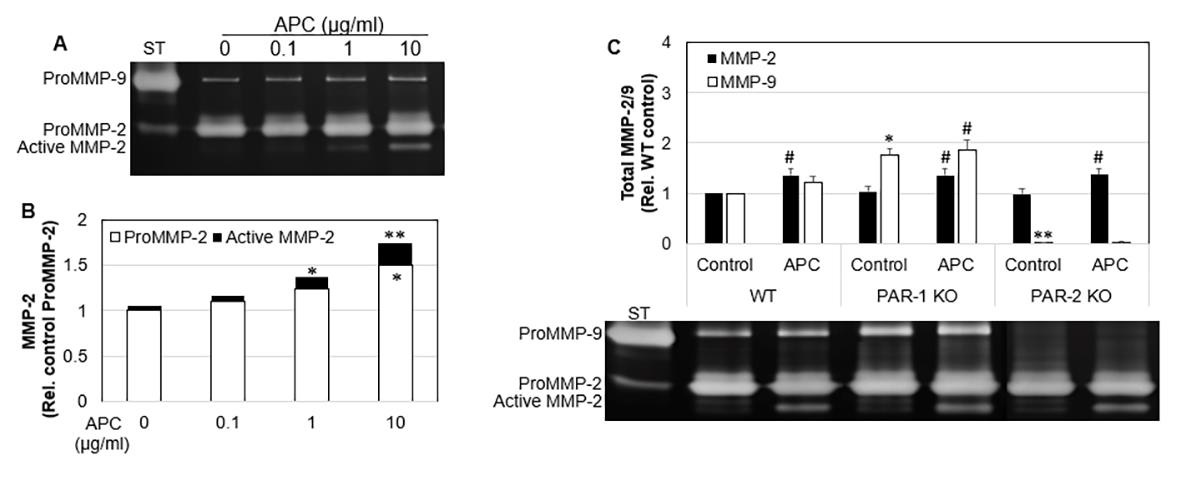
MMP-2 and MMP-9 aid the remodeling phase of healing by contributing to the turnover of collagen and extracellular matrix, and cell migration in many cell types [8,33-35]. Gelatin zymographical data showed that WT tenocytes displayed a dose response increase in MMP-2 expression and activation from 0.1 to 10 μg/mL of APC (Figure 4A). There was a 1.5-fold increase (p<0.05) in MMP-2 expression and 1.7-fold increase (p<0.01) in total MMP-2 at 10 μg/mL APC compared to the control (Figure 4A). APC increased MMP-2 in WT, PAR-1 KO and PAR-2 KO. APC had no effect on WT MMP-9, however, increased MMP-9 expression in PAR- 1 KO cells. PAR-2 KO cells showed decreased MMP-9 expression. These findings show APC increases MMP-2 by PAR-1 and PAR-2, however decreases MMP-9 via PAR-1 and increases MMP-9 via PAR- 2 in murine tenocytes.
Figure 4: The expression and activation of MMP-2 in WT, PAR-1 KO and PAR-2 KO tenocytes in response to APC.
Note: WT, PAR-1 KO and PAR-2 KO Tenocytes were treated with APC for 24 hrs.
A) MMP-2 expression/activation in WT cell culture supernatants, detected by zonography. Data are semi-quantitation by
Image J and depicted in the graph as fold change relative to control.
B) Total MMP-2 and MMP-9 in cell culture supernatants, comparing treatment with APC, detected by zymography. Data are
semi-quantitation by Image J and depicted in the graph as fold change relative to control. Results shown are mean ± SD (n=3)
*p< 0.05 vs WT Control and #p< 0.05 vs their own controls, one-way ANOVA calculated using Tukey post-hoc analysis. **or
##p<0.01.
Discussion
APC is an endogenous serine protease of physiological importance. It has potent anti-coagulant, anti-inflammatory, antiapoptotic and cytoprotective properties. The therapeutic potential of APC has been demonstrated in wide variety of pathologies including sepsis, wound healing, ischemic stroke, lung disorders, kidney injury, diabetic nephropathy, inflammatory bowel disease, systemic lupus erythematosus, amyotrophic lateral sclerosis and cancer metastasis [36-41]. The therapeutic effects of APC on tendon healing have been far less studied. In the only previous study of tenocytes, APC increased tenocyte proliferation, MMP-2 activity, type I collagen deposition and stimulated a healing phenotype in sheep tenocytes [25]. The actions of APC are largely via binding to EPCR, which subsequently activates PAR-1 [42]. However, APC promoted murine skin wound healing via PAR-2, but not via PAR- 1 [43]. Whether and how APC via these receptors mediates the tenocyte healing phenotype has not previously been investigated. In the current study, we demonstrated that deletion of PAR-1 increased tenocyte proliferation, migration and wound healing, whereas deletion of PAR-2 decreased tenocyte proliferation and has no effect on cell migration or wound healing when compared to WT cells; PAR-1 KO and PAR-2 KO cells exhibit increased EPCR expression; APC enhances tenocyte proliferation, migration and wound healing in WT or PAR-1 KO cells, and had limited effect in PAR-2 KO cells. These data indicate that APC promotes tenocyte proliferation and migration by PAR-2, not PAR-1.
Tenocyte proliferation and migration are vital stages of tendon repair [1]. In this study, PAR-1 and PAR-2 displayed differential functions on these two events in mouse tenocytes. This is consistent with a previous study which showed that PAR-2 knockout mice healed significantly slower than wild-type mice and this delayed healing was not altered by adding APC, indicating that APC acts through PAR-2 to heal murine wounds [43]. Similarly, PAR-2 but not PAR-1 modulates synovial macrophage maturation in posttraumatic osteoarthritic mice and thus may play a critical role in the initiation of patient Osteoarthritis [8], and PAR-2 KO mouse synovial fibroblasts exhibited slower rates of proliferation and invasion of than normal cells [9]. These differential functions of PAR-1 and PAR- 2 have also been found in other types of cells or diseases. PAR-1 activation promotes proliferation of human keratinocytes [14] and dermal fibroblasts [15]. It has been shown blocking PAR-1 inhibits APC-induced proliferation in human keratinocytes and thus PAR- 1 appears to promote anti-apoptotic and neuroprotective effects [16,17]. In contrast, PAR-2 affects keratinocyte differentiation, maintains the epidermal barrier, regulates inflammation [18-22] and pain perception [23], and has a tumor-protective role in the skin [24]. In relation to the nervous system, PAR-1 has been shown to mediate mechanisms underlying astrogliosis, vital after brain injury [10].
In regards to the respiratory system, PAR-1 contributes to protective effects of APC on vascular barrier integrity [12] whereas PAR-2 increases ciliary beating which is vital against inhaled pathogens [13]. Additionally, for the cardiovascular system, PAR-1 KO mice have shown an absence of APC’s protective effects against myocardial ischemia/reperfusion injury via inhibition of apoptosis and inflammation [11]. It was found that APC stimulated murine ectopic bone volume and enhances angiogenesis in a model of Bone Morphogenetic Protein 2 induced bone formation. Mechanistically, APC enhances cell proliferation and activates a number of canonical kinase pathways in a PAR1-dependent manner [44]. In comparison, APC induced PAR-1 signaling was shown to regulate the retention and recruitment of EPCR expressing mice bone marrow hematopoietic stem cells [45]. APC was shown to suppress RANKL-induced human osteoclast differentiation mediated through EPCR, PAR-1, S1P receptor, and ApoER2 [46]. While the noted studies above describe different effects of PAR-1 and PAR-2, it must be highlighted that the outcome of PAR activation by the same protease or synthetic agonist can also vary between tissues and cell types [47]. Therefore, the therapeutic role of APC via PARs extends and differs thorough a number of different studies and cell types.
The remodeling stage of tendon healing is predominantly achieved by tissue-degrading enzymes, MMPs [33,48,49]. MMP-9 degrades the ECM days after injury, while MMP-2 participates in both the ECM degradation and remodeling throughout the healing process [34,50,51]. MMP-2 has been associated with increased angiogenesis in in vitro and in vivo studies, however the role of MMP-9 is less distinct [35]. As a result, APC increasing MMP-2/- 9 activity/expression in PAR-1 KO tenocytes may prove vital in stimulating extracellular matrix degradation, angiogenesis and ultimately tendon healing. Other studies [52] have suggested the APC pathway is a potential target for prevention of MMP-2/-9/-13 activation and cartilage extracellular matrix degradation in patients with OA.
The MAPK pathway is essential in regulating key cellular activities including mitogenesis, motility, and survival in many cell types [7]. Although associated with cancer development, distinct MAP kinases including ERK, AKT and GSK-β3 are essential for proliferation and the normal tissue repair process [30-32]. In previous studies, APC has been shown to cleave PARs to elicit cytoprotective effects via numerous signaling pathways, including the MAPK Pathways [29]. In this study, APC stimulated the activation of AKT and GSK in all 3 cell lines, and the activation of ERK in WT and PAR-1 KO cells. This may help explain why PAR- 1 KO mice showed greater cell proliferation. Another study Xue, et al. [25] found that APC dose-dependently stimulated/inhibited ERK/p38 signaling in sheep tenocytes at 24 hours, respectively. It is feasible that MAP kinase signaling in response to APC is different in different species and at different time frames post treatment, being 1hr in our study.
Since tendinopathy can be both hyper- or hypo-cellular [53], fine-tuning the balance between APC’s interaction between PAR-1 and PAR-2 could help regulate tenocyte equilibrium. These finding could be vital as a large body of evidence suggests the promise of regenerative medicine will be achieved by focusing on augmenting the natural healing response, where little is known about the synergistic and antagonistic interactions of growth factors and perhaps their receptors to produce the best effects [54,55].
Conclusion
We demonstrated that depletion of PAR-1 increased tenocyte
proliferation, migration and wound healing, whereas depletion
of PAR-2 only decreased cell proliferation and had no impact
on cell migration or wound healing when compared to WT cells.
Depletion of PAR-1 or PAR-2 increased EPCR expression, but has
no effect on either PAR-2 or PAR-1 expression. APC enhanced
tenocyte proliferation only in WT or PAR-1 KO cells, not in PAR-2
KO cells; promoted migration and wound healing in PAR-1 KO cells;
and increased migration only in PAR-2 KO cells. These findings
suggest that APC promotes tenocyte proliferation, migration and
wound healing largely by PAR-2, and not PAR-1. APC increases
MMP-2 by PAR-1 and PAR-2, however, decreased MMP-9 via PAR-
1 and increases MMP-9 via PAR-2 in murine tenocytes. Overall,
APC promotes a healing phenotype in cultured murine tenocytes
largely via PAR-2, no PAR-1. By shedding light on APC’s therapeutic
mechanistic action through PARs, this work has laid the initial
platform in maximizing APC’s potential in improving tenocyte
healing and treating tendinopathy.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.