MORUS RUBRA L: “Phytochemical Screening, Anti- Bacterial, Antioxidant and Anti-Hyperglycemic Activity Determination
Introduction
Morus rubra Linn (MR), generally known as red mulberry belong to the Moraceae,
mulberry family. It is originated from the eastern part of the United
States of America and is now universally cultivated and naturalized in
the areas of China, Korea and Japan. Edible fruits that are reddishly
maturing to dark purple or black in colour. The fruits are soft, juicy
and aggregate with a sweet taste and a hint of sourness, which is more
evident in the less mature fruits. In addition, red mulberry sap was
used by several tribes to treat ringworm while the stem bark is used as
purgative and vermifuge. The fruits have also been utilized to treat
diabetes, hypertension, anaemia and arthritis in traditional medicine,
particularly in Chinese medicine [1] (Figures 1-4). It is also
demonstrated that Morus rubra (MR) fruit leads to control over
hyperglycaemia and may be a good antimicrobial agent against gram
negative bacterial infections. The first documented use of mulberry
fruits was described in the mid-1500s by De Soto expedition, who
discovered consuming these dried fruits [2]. Diabetes is a heterogeneous
disease affecting almost 6% of the world population. It is
characterized by hyperglycemia and insulin resistance or a combination
of both of these factors.
The treatment of diabetes with allopathic drugs causes moderate to severe adverse events. Hence, the alternative systems of medicine from plant and marine sources are being explored to treat diseases [3] (Tables 1-4). Fully ripened mulberry fruit has a delicious, mouth-watering taste with a pleasant aroma and flavor. It is valued for both direct consumption and the creation of value-added products. Mulberry fruits are known for their nutritional value, which makes them beneficial to human health. Principal sugars present in mulberry are fructose and glucose, which increase with ripening [4,5]. Whereas the primary fatty acid that found in the mulberry fruit are oleic acid, palmitic acid and linolenic acid [6,7]. Citric acid, tartaric acid, malic acid, succinic acid and fumaric acid are among the organic acids found in mulberry fruits; nonetheless, malic acid is the most abundant organic acid present. Mulberry is also high in a number of essential minerals including calcium, phosphorus, potassium, magnesium and sodium.
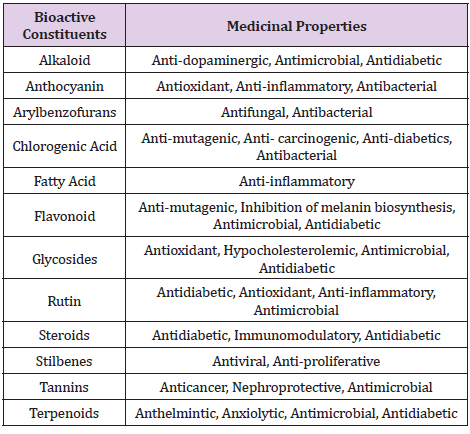
Table 4: Red Mulberry (MR) with Pharmacological Actions. (Elbaz, et al. [10,12-14]) (Thabti et al., 2020) (Wei et al., 2018).
Materials and Methods
The raw material was collected and purchased from a loca Indian market. sun dried at a temperature of 30˚C under the shade for several days to eliminate the excessive moisture content. The dried sample was preserved in a dry container with low humidity conditions to prevent the absorption of the moisture by the dried sample. The dried sample was ground into powder grinding machine. The extraction process started by placing the dried sample and solvent together in a vessel or extraction instrument. The extractions of MR were carried out with ethanolic Soxhlet extraction and maceration.
Chemicals / Equipment
Nutrient Agar (HiMedia Laboratories Pvt.Ltd. India), Nutrient Broth (HiMedia Laboratories Pvt.Ltd. India), Tryptic Soy Agar (HiMedia Laboratories Pvt.Ltd. India), Tryptic Soy Broth (HiMedia Laboratories Pvt.Ltd. India), Ciprofloxacin, Penicillin,70% Alcohol, Plant Extract and Dimethyl Sulfoxide (DMSO) (Fisher Scintific UK). Ethanol 95% (John Kollin Corporation, USA), Methanol (J.T. Baker. Center Valley, PA), α,αdiphenyl-β-picrylhydrazyl (DPPH) (Sisco Research Laboratories Pvt. Ltd, India.), Butyl Hydroxyl Toluene (BHT), Sodium Carbonate (Bendosen Laboratory Chemicals), Folin- Ciocalteu’s Phenol Reagent (EMD M.C. Germany), Gallic Acid (R&M Chemicals, UK), Shimadzu UV- Visible Spectrophotometer.
Bacterial Strains
The cultures were used for the antibacterial assay. These
cultures were obtained from microbial culture bank of Faculty of
Pharmacy (FOP), Aimst University.
a) Escherichia coli (E. coli) ATCC 8739.
b) Staphylococcus aureus (S. aureus) ATCC 29737.
c) Bacillus subtilis (B. subtilis) ATCC 6633.
d) Salmonella typhi (S. typhi) ATCC 19430.
Maceration Extraction
510gm of mulberry ground fruits were weighed and soaked in 1 L of 95% ethanol. The conical flask was kept at dark, in the cupboard for 9 days and swirling was done on daily basis throughout the maceration process. After 9 days, the maceration process was completed and the mixture was filtered by using muslin cloth. The volume of maceration extract collected from this process was 850 ml. The maceration extract was evaporated using rotary evaporator (Rotary evaporated R-210 BUCHI, Corporation) at 75°C and the speed of the rotator was set to 100 rpm. The final volume of maceration semi solid product collected from this process was 300 ml.
Soxhlet Extraction
450 gm of fruits ground powder were weighed and 1L ml of 95 % ethanol was poured into the soxhlet flask. The heating mantle was kept at 80 ºC. The duration for this Soxhlet extraction process to be completed was about 17hours. Decolorization of purple colour of the fruits was observed. The total volume of Soxhlet extract collected from this process was 350 ml. The extract was evaporated using rotary evaporator at 75°C and the speed of the rotator was set to 100 rpm. The extract was evaporated using rotary evaporator (Rotary evaporated R-210 BUCHI, Corporation) at 75°C and the speed of the rotator was set to 100 rpm. The final volume of product collected from this process was 250 ml. Figures 5 & 6 shows the evaporated extracts for maceration and hot extraction respectively.
Phytochemical Analysis
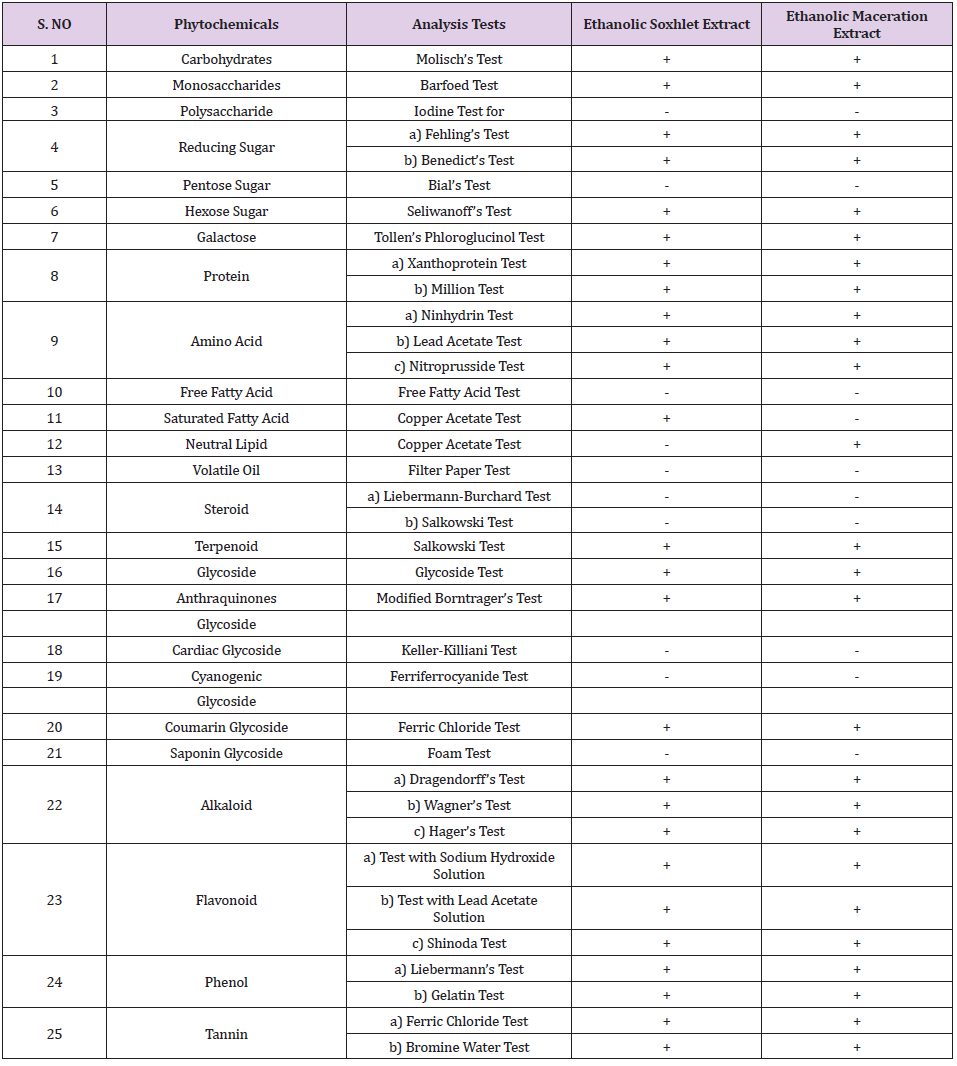
Phytochemical tests were carried out to identify the presence of carbohydrates, monosaccharides, polysaccharide, reducing sugar, pentose sugar, hexose sugar, galactose, protein, amino acid, free fatty acid, saturated fatty acid, neutral lipid, volatile oil, steroid, terpenoid, glycoside (anthraquinones glycoside, cardiac glycoside, cyanogenic glycoside, coumarin glycoside, saponin glycoside), alkaloid, flavonoid, phenol and tannin. The total phenolic content of MR using 50 and 200μg/ml solution was determined by Folin– Ciocalteu reagent method using gallic acid as standard [8].
Antioxidant Assay
Antioxidant activity of ethanolic extract of MR is studied using DPPH method. The DPPH is a stable free-radical which has a colour of purple, as the antioxidants react with the DPPH, the DPPH will be converted into non-radical DPPH-H form. Besides that, the colour of the DPPH will be decolorized from purple to light yellow colour. The degree of decolorization indicates the potency of the plant extracts in scavenging the free radicals. 0.1M of methanolic DPPH was freshly prepared by dissolving 3.94mg of DPPH crystalline powder in 100ml of methanol and kept in a clean beaker. Extracts (0.2 ml) at different concentrations (50–1000 μg/ml) was mixed with 0.8 ml of tris hydrochloric acid (HCl) buffer (100 mM; pH 7.4). One millilitre DPPH (500 mM in 1.0 ml ethanol or methanol) solution was added to the mixture. The mixture was shaken vigorously and incubated for 30 min at room temperature. The absorbance of the resulting solution was measured spectrophotometrically at 518 nm (Model UV 1800, Shimadzu, Japan). BHT is used a standard. All the determinations were carried out in triplicate. The following formula was used for the antioxidant activity determination [9]. DPPH Radical Scavenging Activity (%) = 𝐴𝑐𝑜𝑛𝑡𝑟𝑜𝑙−𝐴𝑠𝑎𝑚𝑝𝑙𝑒𝐴𝑐𝑜𝑛𝑡𝑟𝑜𝑙 𝑥 100.
Antibacterial Assay
The 10 mg/ml stock solution of ethanolic extract of MR is prepared using 0.5% DMSO (prepared using distilled water). The antimicrobial activity of ethanolic extract of MR against four pathogenic microorganisms (namely Escherichia coli [ATCC 8739], Staphylococcus aureus [ATCC 29737], Bacillus subtilis [ATCC 6633], Salmonella typhi [ATCC 19430]) was carried out. Using stock, the primary culture was prepared and used for preparation of sub-cultures of microorganism. The antibacterial activity was tested by agar well diffusion method. E. coli, S. aureus, B subtilis and S. typhi were spread onto the surface of nutrient agar with sterile swab (0.1 ml containg106 cell/ml). Six mm diameter wells were punched into the agar and filled with 0.1ml of the ethanolic extract or standards (penicillin and ciprofloxacin). Then incubated at 37ᵒC for 24 hours. After 24 hours, zone of inhibition were measured. All experiments were carried out in triplicates.
Minimum Inhibitory Concentration (MIC) is determined by using the broth dilution method. Serial dilution was made between 1000 mg/ml and 62.5 mg/ml. Mueller- Hinton (MH) broth was prepared and 1ml was added into test tubes alongside 100μl of selected ATCC strains. Then, 1ml of extracts with the concentrations mentioned above were introduced into the test tubes contained the broth and ATCC strain and mixed for even distribution. These test tubes were then incubated for 36 hrs at 37°C. As a positive control, 1 ml of selected antibiotics was added to the test tube while the negative control only contained selected ATCC strains [10]. The results obtained after incubation were compared to both the positive and negative control. The concentration of the extract that gave clear results (absence of turbidity) was considered as the MIC of the extract. The Minimum Bactericidal Concentration (MBC) was determined as the lowest concentration of extract that killed at least 99% of the initial bacterial number [11].
Antidiabetic Activity
Healthy, adult, Sprague-Dawley (SD) rats (180 ± 20 g) were
used for the studies. The animals were obtained from Central
animal house, AIMST University, Malaysia. Approval from the
AIMST University Human and Animals Ethics Committee was
obtained. The animals were housed in large, spacious polyacrylic
cages at ambient room temperature with 12 h light/12 h dark cycle.
A minimum of 5 days of acclimatization period was allowed before
the animals are used in the experiment. The animals were fed with
water and normal rodent pellet diet ad libitum. Diabetes mellitus
will be induced in overnight-fasted rats by administration of single
intraperitoneal (I.P.) injection of freshly prepared streptozotocin
(STZ) with a dose of 60 mg/kg/mL [12]. To prevent the STZ-induced
hypoglycaemia, rats administered with 10% dextrose solution after
24 h of STZ administration for next 24 h. Induction of diabetes
was verified after 72 h by measuring blood glucose level with
strips using glucometer and the animals allowed 14 days for the
stabilization of blood glucose level [13]. On day 14, animals having
a blood glucose level higher than 220 mg/dL were considered
diabetic and included in the experiments. Diabetic animals were
randomly divided into five groups (Group II–VI) as follows:
Group I: Normal control
Group II: Diabetic control
Group III: Diabetic animals treated with glibenclamide (20 mg/
kg/P.O.)
Group IV: Diabetic animals treated with ethanolic extract of MR
(100 mg/kg/P.O.) Group V: Diabetic animals treated with ethanolic
extract of MR (200 mg/kg/P.O.)
Group I (normal control) and group II (diabetic control) rats
were given 0.5% w/v carboxymethylcellulose (CMC) while the
rats in group III treated with 20mg/kg body weight (BW) of
glibenclamide and rats in group IV-V were administered with
ethanolic extract of MR of 100mg and 200mg per kg. The standard
and test drugs were suspended in 0.5% w/v CMC and administered
once daily through oral gavage for 28 consecutive days. Throughout
the study, variations in experiment animals’ body weight will be
monitored at regular intervals. At the end of the study, the blood
samples withdrawn from all the experimental rats through retroorbital
plexus puncture. The serum was separated from the blood
sample and used for biochemical analysis.
Statistical Analysis
The value in the antidiabetic activity was expressed as mean ± Standard Error of the Mean (SEM). The data was analysed using one-way analysis of variance (ANOVA), followed by Tukey’s posthoc test. P < 0.05 will be considered as statistically significant.
Results
Phytochemical analysis showed the presence of carbohydrates, monosaccharides, reducing sugar, hexose sugar, galactose, protein, amino acid, neutral lipid, terpenoid, glycoside, anthraquinones glycoside, coumarin glycoside, alkaloid, flavonoid, phenol and tannin. The phytochemical screening is shown in Tables 5 & 6. The total phenolic content found was 6.420 mg GAE/g and 6.097 mg GAE/g for the concentrations of 50 and 200µg/ml respectively. Antioxidant activity of ethanolic extract of MR is studied using DPPH method and BHT was used as standard. In DPPH radical scavenging method, BHT and MR extract showed 50% inhibition (IC50) at 84.04µg/ml and 182.82µg/ml, respectively. The antibacterial activity of ethanolic extract of MR was tested by using the E. coli, S. aureus, S. typhi, and B. subtilis. The extract concentrations used in the agar well diffusion method were 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml, other than that, 5mg/ml of penicillin and ciprofloxacin was playing the role as a standard in this study. In the present study, there was no zone of inhibition observed in all concentrations of ethanolic MR extract. The microorganism used were all in active condition, this can be proven as the ciprofloxacin as well as penicillin showed a clear zone of inhibition.
Table 5: Results of Phytochemical Screening of MR.
Note: Positive results are denoted by (+) sign, negative results are denoted by (-) sign.
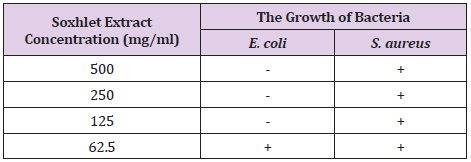
In antimicrobial screening, ethanolic extract of MR exhibited antibacterial activity against E. coli with equivalent MIC and MBC identified at the concentration of 500 mg/ml, 250 mg/ml and 125 mg/ml and did not inhibit S. aureus. Anti-hyperglycaemic activity of ethanolic extract of MR was studied using STZ-induced diabetic rats. The diabetic animals were showed decreases in body weight but the results were not significant. The diabetic rats showed significant increases in the levels of glucose throughout the study when compared with that of control animals. Whereas the diabetic animals treated with glibenclamide or ethanolic extract of MR (100/ 200 mg/kg) showed significant decreases in the glucose levels when compare with that of diabetic control animals.
Antimicrobial Screening
The antimicrobial activity of Morus Rubra (MR) ethanolic Soxhlet fruit extract was analyzed against the gram negative bacteria Escherichia coli and gram positive bacteria Staphylococcus aureus at 4 different concentrations: 500 mg/ml, 250 mg/ml, 125 mg/ml and 62.5 mg/ml. It was expressed by identifying the MIC and MBC of fruit extract through the broth tube dilution method. The obtained result from the MIC test showed that turbidity was observed in all the assay tubes that tested against the S. aureus. Therefore, three tubes with the least turbidity were selected and subjected for MBC.
Preparation of Tryptic Soy Agar Plate
Tryptic soy broth or trypticase soy broth (frequently abbreviated as TSB) is used as a culture broth to grow aerobic bacteria. It is a complex, general purpose medium that is routinely used to grow certain pathogenic bacteria, which tend to have high nutritional requirements (i.e., they are fastidious). Its agar counterpart is Tryptic Soy Agar (TSA). One of the components of Tryptic soy broth is Phytoene which is an enzymatic digest of soybean meal). TSB is frequently used in commercial diagnostics in conjunction with the additive sodium thioglycolate which promotes growth of anaerobes. Tryptic Soy Agar was only applied for the Bacillus subtilis out of the 4 chosen bacterial strains. 20g of tryptic soy agar powder was weighed and transferred into 500ml of distilled water. The conical flask was subjected to sterilization with the use of autoclave for 2 hours at the temperature of 121˚C. In the biosafety cabinet, the freshly prepared sterilized tryptic soy agar was poured into the sterilized Petri plates. The inoculation loop was used to eliminate the bubble formed on the surface of the tryptic soy agar before solidification occurs. Approximately, 25ml of the tryptic soy agar was poured into the sterilized Petri plates, these plates were allowed to cool down and solidified in the biosafety cabinet. These plates were incubated in an upside-down position at the temperature of 37˚C and were taken out during the agar well diffusion test.
Agar Well Diffusion
Before the initiation of the agar well diffusion test, the biosafety cabinet was sterilized with 70% alcohol and the UV light was switched on for 10 minutes. Apart from that, the plant extract dilutions, antibiotic dilutions with the concentration of 5mg/ml (Penicillin and Ciprofloxacin), bacteria strains, bacteriological loop, sterilized cork borer, sterilized micropipette tips and micropipette (p100), Bunsen burner and nutrient agar plate were prepared in prior of the test. After switched off the UV light and placement of all materials in the biosafety cabinet, gloves were worn and 70% alcohol was applied properly to sanitize the gloves to prevent contamination occurs. NA/TSA (nutrient agar/tryptic soy agar), date, name, bacterial strain, ATCC code and antibiotics were labelled accordingly at the bottom side of the plates. 0.1 ml of broth that contained bacterial strain was pipetted into the Petri plates with the use of a micropipette. The sterilized glass spreader was used to spread the bacterial strains evenly on the plate. After every spreading, the glass spreader should be sanitized with 70% alcohol and showed to the flame.
Then the sterilized cork borer was used to make wells on the agar plate. There were 4 wells punched in one Petri plate, each well was separated from each other in equal distance. The plates were prepared in a triplicate manner for the comparison purpose, as well as the positive and negative plates. The cork borer was sterilized every time after use. The agar which was fitted inside the cork borer after punching the wells was discarded. The different concentrations of plant extract as well as different antibiotics were added into the wells by using a micropipette. The wells were filled until 60% of the height of the wells. The micropipette tips were discarded after each concentration of plant extract and antibiotics was performed. This can prevent an alteration of the concentration of plant extract and antibiotics. The Petri plates were then incubated in an incubator at the temperature of 37˚C for 24 hours. The zone of inhibition of the plates was observed and recorded.
Minimum Inhibitory Concentration (MIC)
5 loops of the bacterial strains were cultured in each sterile nutrient broth and then incubated at 37 ͦ C for 24 hours, centrifuged at 5000 rpm for 10 minutes.
Preparation of Standard McFarland Bacterial Culture: The nutrient broth as well as tryptic soy broths were prepared freshly. These broths were sterilized for 2 hours at the temperature of 121˚C and were allowed to cool down. In the biosafety cabinet, 5 loop full of Bacillus subtilis bacteria colonies were inoculated into the tryptic soy broth. This step was repeated for the other three bacteria which were inoculated in the nutrient broth. These broths were incubated for 24 hours at the temperature of 37˚C in an incubator. Then, these broths that contained bacteria strains were centrifuged at 5000rpm for 10 minutes. The supernatant was discarded and the cell pellet formed was collected and re-suspended in the sterile nutrient broth and tryptic soy broth. By using the UV spectrophotometer, the seeded broths were standardized to the value 0.5 of the absorbance according to McFarland standard at the wavelength of 517nm.
Preparation of Sample: The extracts were obtained from the Soxhlet extraction method using 95% ethanol. 4 different concentrations of the extracts were prepared at 25, 50, 100, 200μg/ml. 2 mg of extracts was dissolved in 10ml of ethanol, forming a stock solution with a concentration of 200μg/ml. Then it was double-diluted by mixing 2ml of the stock solution and 2ml of ethanol to obtain the concentration of 100μg/ml. The same procedure was repeated and obtained 50μg/ml in concentration. Next, 1ml of stock solution was taken and mixed with 2ml of ethanol to obtain a concentration of 25μg/ml. 4 test tubes were prepared and 0.2ml of the sample solution were taken and transferred into the test tube prepared. 4ml of 2.5% sodium carbonate and 0.2ml of Folin-Ciocalteu reagent was added to each of the test tubes. The test tubes were allowed to sit for 2 hours. The absorbance of the standard solution was examined by 750nm UV spectrophotometry and was recorded.
Preparation of Blank: 0.2ml of Folin-Ciocalteu reagent, 4ml of 2.5% sodium carbonate and 0.2ml of ethanol were added into the test tube and it was allowed to sit for 2 hours. The absorbance of the standard solution was examined by 750nm UV spectrophotometry. The values of absorbance of standard and sample obtained were then utilized in plotting the graph of UV absorbance against the concentration of sample and standard.
Serial Dilution of Minimum Inhibitory Concentration (MIC) Assay
Before starting the MIC assay, the biosafety cabinet was sanitized with 70% alcohol and the UV light was switched on for 10 minutes. 2.5 g of plant extract was measured using an analytical balance and mixed with 25ml of 0.5% DMSO (dimethyl sulphoxide), forming the stock solution with a concentration of 10mg/ml. The mixtures with the concentration of 50, 25, 12.5, 6.25, 3.125, 1.5625 mg/ml were formed upon two-fold serial dilution. 2 ml of the stock solution and 2ml of the standard McFarland seeded broth were taken by using a micropipette and mixed in the sterilized test tube, thus obtained a concentration of 50mg/ml. 2 ml of the stock solution and 2ml from the first test tube were taken by using a micropipette and mixed in the sterilized test tube, thus obtained a concentration of 25mg/ ml. 2 ml of the stock solution and 2ml from the second test tube were taken by using a micropipette and mixed in the sterilized test tube, thus obtained a concentration of 12.5mg/ml. 2 ml of the stock solution and 2ml from the third test tube were taken by using a micropipette and mixed in the sterilized test tube, thus obtained a concentration of 6.25mg/ml. 2 ml of the stock solution and 2ml from the fourth test tube were taken by using a micropipette and mixed in the sterilized test tube, thus obtained a concentration of 3.125mg/ml. 2 ml of the stock solution and 2ml from the fifth test tube were taken by using a micropipette and mixed in the sterilized test tubes.
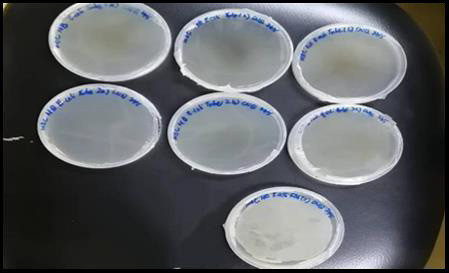
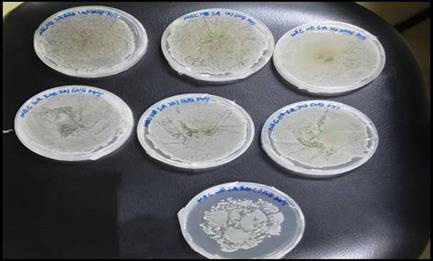
2ml of the mixture was pipetted and discarded, thus obtained a concentration of 1.5625mg/ml. The positive tube that contained seeded broth and the negative tube that contained sterile nutrient or tryptic soy broth were prepared accordingly. The micropipette tips were discarded after finished transferring each set of test tubes. These tubes were fitted with cotton wool and covered with double-folded aluminium foil the tubes were incubated for 24 hours at the temperature of 37˚C. The turbidity of each of the tubes were observed and recorded. In contrast, the extract that tested against the E. coli showed clear solution at concentration of 500 mg/ml, 250 mg/ml and 125 mg/ml. This indicated that ethanolic Soxhlet extract of Morus rubra did not exhibit antibacterial action against E. coli at these concentrations. Hence, these three assay tubes were subculture on fresh sterilized nutrient agar plates to find out the MBC of the fruit extract on E. coli. From the MBC analysis, the nutrient agar plates for the S. aureus showed the bacterial growth revealed that the ethanolic fruit extract had bacteriostatic or bactericidal effect against S. Aureus. However, positive results were obtained for the E. coli in which there is no observed bacterial growth on all three nutrient agar plates. Therefore, the fruit extract possessed equivalent MIC and MBC on E. coli, ranged from 125 mg/ ml to 500 mg/ml. These results suggested that ethanolic Morus rubra extract was more susceptible to the gram positive bacteria than gram negative bacteria. The antibacterial properties of the red mulberry may be due to the presence of phenolic compounds in abundance such as flavonoid. At a low concentration, the phenols were acted as bacteriostatic and at a high concentration, phenols may act as bactericidal. The results are shown in Table 7. MCB results are laid down in Figures 7 & 8.
Figure 7: MBC result of triplicate sets E.Coli and a positive control plate. There is no observed bacterial growth on the agar plate excluded the positive control plate.
Figure 8: MBC result of triplicate sets S.aureus and a positive control plate. The bacterial growth was observed on all the agar plates.
Antioxidant screening
The MR were cleanly washed and air-dried at room temperature for 1 week to prevent deterioration. MR were passed through a sieve 1.40 mm to remove the unwanted impurities and then pulverized into powder form using the mortar and pestle. The extraction of the seeds was carried out using ethanol as an extracting solvent. The Soxhlet extraction method was employed to extract the chemical constituents in the MR. 19.38 gm of the MR powder was inserted into a thimble 300 ml of ethanol was measured accurately and added into the round bottom flask. The running of tap water has remained until the whole apparatus cools down. The MR extract contained in the round bottom flask was poured into a cleaned and dried conical flask (1000 ml). The conical flask was covered with cotton wool and aluminium foil to prevent evaporation. The ethanolic extract of MR was evaporated by using the rotary evaporator. The temperature of the rotary evaporator was set at 80˚C. The process was continued until no notable changes were observed. The MR extract was poured into a clean beaker. The collected pure extract was introduced into a China dish and placed on the hot water bath for further evaporation.
Determination of total Phenolic Content Preparation of Standard
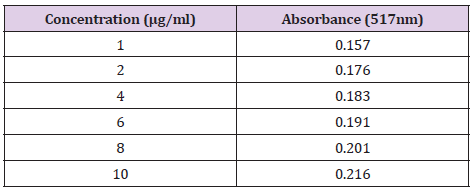
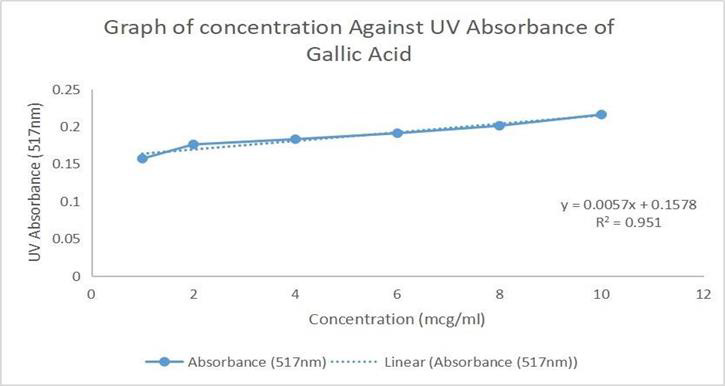
Gallic acid was used as a standard in this assay and compared with the sample to identify the presence of phenolic compounds in the plant extract. Five different concentrations of Gallic acid were prepared at 1, 2, 4, 6, 8 and 10μg/ml. The stock solution was prepared by dissolving 10mg of Gallic acid in 10ml of 95 % ethanol. Then, 1ml of the solution was diluted with 9ml of ethanol to obtain a concentration of 100μg/ml. The same procedure was repeated until the final concentration of 1μg/ml was obtained. From the stock solution, 1.0, 0.8, 0.6, 0.4, 0.2, 0.1ml were pipetted into 6 different tubes and labelled accordingly. The volume was then made up to 10ml with 95% ethanol. Another 6 clean tubes were prepared. 0.2ml of the standard solution were taken and transferred into the test tube prepared. 4 ml of 2.5% sodium carbonate and 0.2ml of Folin-Ciocalteu reagent was added to each of the test tubes. The test tubes were allowed to sit for 2 hours. The absorbance of the standard solution was examined by 750nm UV spectrophotometry and was recorded.
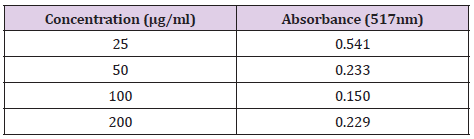
Preparation of Sample: Four different concentrations of the extracts were prepared at 25, 50, 100, 200μg/ml. 2 mg of extracts was dissolved in 10ml of ethanol, forming a stock solution with a concentration of 200μg/ml. Then it was double-diluted by mixing 2ml of the stock solution and 2ml of ethanol to obtain the concentration of 100μg/ml. The same procedure was repeated and obtained 50μg/ml in concentration. Next, 1ml of stock solution was taken and mixed with 2ml of ethanol to obtain a concentration of 25μg/ml. 4 test tubes were prepared and 0.2ml of the sample solution were taken and transferred into the test tube prepared. 4ml of 2.5% sodium carbonate and 0.2ml of Folin-Ciocalteu reagent was added to each of the test tubes. The test tubes were allowed to sit for 2 hours. The absorbance of the standard solution was examined by 750nm UV spectrophotometry and was recorded.
Preparation of Blank: 0.2ml of Folin-Ciocalteu reagent, 4ml of 2.5% sodium carbonate and 0.2ml of ethanol were added into the test tube and it was allowed to sit for 2 hours. The absorbance of the standard solution was examined by 750nm UV spectrophotometry. The values of absorbance of standard and sample obtained were then utilized in plotting the graph of UV absorbance against the concentration of sample and standard.
DPPH Radical Scavenging Assay (diphenyl-picrylhydrazyl hydrate)
Preparation of DPPH Solution: 0.1mM of methanolic DPPH was freshly prepared by dissolving 3.94mg of DPPH crystalline powder in 100ml of methanol and kept in a clean beaker. The beaker was covered with aluminium foil to prevent oxidation.
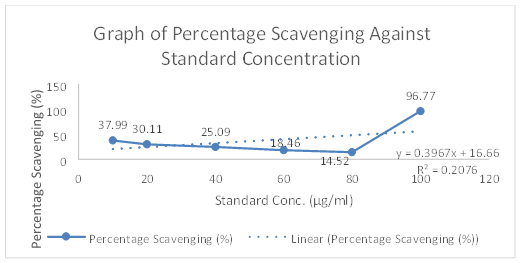
Preparation of Standard: BHT was used as a standard in this assay. Six different concentrations of BHT solutions were prepared at 10, 20, 40, 60, 80 and 100μg/ml. The stock solution was first prepared by dissolving 10mg of BHT in 10ml of methanol. 1ml of the solution was then diluted ten-fold with 9ml of methanol to obtain a concentration of 100μg/ml. From the stock solution, 10, 8, 6, 4, 2, 1ml were pipetted into 6 different test tubes and made up to 10ml with methanol. Then, 3ml of 0.1mM DPPH reagent was added to 2.5ml of different concentrations of methanolic extracts. The mixtures were shaken and labelled accordingly. The test tubes were allowed to keep in the dark for 30 minutes. The absorbance was measured at 518nm using a UV spectrophotometer.
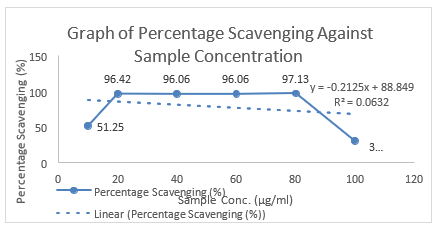
Preparation of Sample: The steps in preparation of DPPH solution were repeated in the preparation of the sample, in which the BHT was changed to the plant extract. The absorbance was measured at 518nm using a UV spectrophotometer.
Preparation of Blank: The blank solution was prepared by mixing 3ml of 0.1mM DPPH reagent and 2.5ml of methanol, the solution was allowed to stand in the dark for 30 minutes. The absorbance was measured at 518nm using a UV spectrophotometer. The values of absorbance of standard and sample obtained were then utilized in plotting the graph of UV absorbance against concentration of the sample and standard.
Antibacterial Assay
Sterility Test of Ethanolic MR Extract
Before the initiation of phytochemical screening, antibacterial and antioxidant assay of MR extract. The extract was streaked on nutrient agar and was incubated for 24 hours at the temperature of 37˚C. Due to the movement control order in Malaysia, the plant extract was kept in the fridge for about 3 months, therefore the growth promotion test is able to determine whether microbes were present in the plant extract. This enables us to ensure positive results for all tests, especially antibacterial assay. There was no bacterial growth observed after 24 hours of incubation.
Serial Dilution of Extract: Before the dilution of plant extract, the 0.5% DMSO was prepared by measured 0.5ml of DMSO and mixed with 99.5ml of sterilized distilled water. 400mg of plant extract was weighed with the use of analytical balance and it was transferred to a clean beaker that contained 40ml of 0.5% DMSO. This gave rise to the formation of a stock solution with a concentration of 10mg/ml. Next, the beaker was subjected to sonication to ensure complete mixing of the two different liquid. From the stock solution, 10, 8, 6, 4, 2, 1ml were pipetted into 6 different sterilized universal tubes and were labelled accordingly. The volume was then made up to 10ml with 0.5% DMSO. The universal tubes were tightly screwed and kept in the refrigerator for storage.
Preparation of Bacterial Strains: 1.95g of nutrient broth powder and 1.5g of tryptic soy broth powder were weighed accurately using an analytical balance and transferred into 4 different conical flasks (100ml). These conical flasks were labelled accordingly.
Preparation of Nutrient Agar Plate: 11.2g of nutrient agar powder was weighed 3 times and transferred into 3 conical flasks (1000ml). 400ml of distilled water was measured accurately and poured into each of the flasks. After putting magnetic beads into 3 of the conical flasks, these conical flasks were placed on the magnetic stirrer to facilitate the dissolving process. The mouth of the conical flasks was fitted with cotton wool and covered with double-folded aluminium foil. The conical flasks were subjected to sterilization with the use of autoclave for 2 hours at the temperature of 121˚C. In the biosafety cabinet, the freshly prepared sterilized nutrient agar was poured into the sterilized Petri plates. The inoculation loop was used to eliminate the bubble formed on the surface of the nutrient agar before solidification occurs. Approximately, 25ml of the nutrient agar was poured into the sterilized Petri plates, these plates were allowed to cool down and solidified in the biosafety cabinet. These plates were incubated in an upside-down position at the temperature of 37˚C and were taken out during the agar well diffusion test.
Preparation of Tryptic Soy Agar Plate: Tryptic soy broth or Trypticase soy broth (frequently abbreviated as TSB) is used as a culture broth to grow aerobic bacteria. It is a complex, general purpose medium that is routinely used to grow certain pathogenic bacteria, which tend to have high nutritional requirements (i.e., they are fastidious). Its agar counterpart is tryptic soy agar (TSA). One of the components of Tryptic soy broth is Phytoene which is an enzymatic digest of soybean meal). TSB is frequently used in commercial diagnostics in conjunction with the additive sodium thioglycolate which promotes growth of anaerobes. Tryptic Soy Agar was only applied for the Bacillus subtilis out of the 4 chosen bacterial strains. 20g of tryptic soy agar powder was weighed by using an analytical balance and transferred into a 1000ml conical flask.
500ml of distilled water was poured into the conical flask and labelled accordingly. After putting the magnetic bead into the conical flask, the conical flask was placed on the magnetic stirrer to facilitate the dissolving process. The mouth of the conical flask was fitted with cotton wool and covered with double-folded aluminium foil. The conical flask was subjected to sterilization with the use of autoclave for 2 hours at the temperature of 121˚C. In the biosafety cabinet, the freshly prepared sterilized tryptic soy agar was poured into the sterilized Petri plates. The inoculation loop was used to eliminate the bubble formed on the surface of the tryptic soy agar before solidification occurs. Approximately, 25ml of the tryptic soy agar was poured into the sterilized Petri plates, these plates were allowed to cool down and solidified in the biosafety cabinet. These plates were incubated in an upside-down position at the temperature of 37˚C and were taken out during the agar well diffusion test.
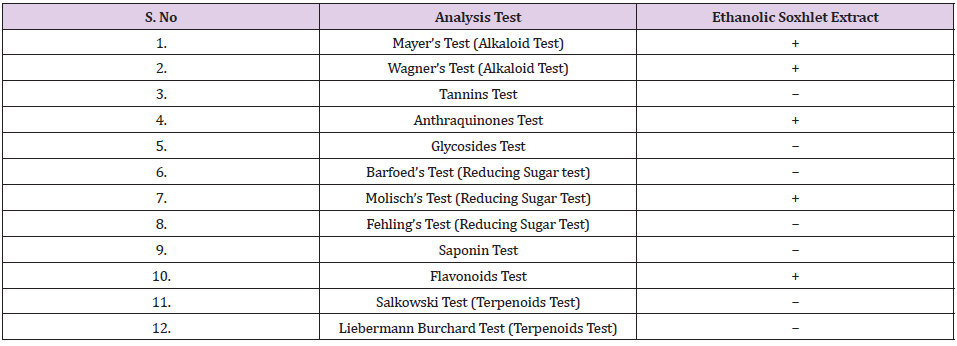
Phytochemical Screening of Ethanolic MR Extract: The MR was extracted with 95% ethanol, and the plant extract was undergone phytochemical screening. In the present study, the results obtained for the phytochemical screening of MR ethanolic extract was analysed and it was found out that alkaloids, anthraquinone, carbohydrate and flavonoids were present in the sample. The alkaloid content was tested with Mayer’s and Wagner’s reagent. Mayer’s reagent is an alkaloidal precipitating reagent which its function is to determine the presence of alkaloids in natural products. Most alkaloids will be precipitated in a neutral or slightly acidic solution owing to Mayer’s reagent. Cream colour precipitate will be obtained upon observation. The ethanolic extract of MR showed positive results for both Mayer’s and Wagner’s tests, which indicates the MR contained alkaloids component.
Antioxidant Assay
Determination of Total Phenolic Content (TPC): In this research, the antioxidant potential of the MR was determined by using DPPH free radicals scavenging method and BHT was used as a standard. The DPPH is a stable free-radical which has a colour of purple, as the antioxidants react with the DPPH, the DPPH will be converted into nonradical DPPH-H form. Besides that, the colour of the DPPH will be decolorized from purple to light yellow colour. The degree of decolorization indicates the potency of the plant extracts in scavenging the free radicals.
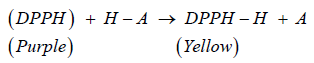
Subsequently, the plant extract was prepared in the concentration of 10, 20, 40, 60 , 80 and 100 μg/ml and mixed with the DPPH, the absorbance was determined at 518nm after kept the tubes in the dark for 30 minutes. Phenolic compounds are very essential plant constituents which responsible for the antioxidant activity due to its redox properties. As a basis, Folin-Ciocalteu reagent was used to measure the phenolic content of different concentrations of plant extract. In this test, the Gallic acid was used as a standard and the total phenolic content was calculated by using the formula C = (A/B) x dilution factor. The results were derived from the calibration curve of Gallic acid, it was expressed as Gallic acid equivalents (GAE) per gram of samples. In the present study, the total phenolic content found was 6.420 mg GAE/g and 6.097 mg GAE/g for the concentrations of 50 and 200μg/ml respectively. The following formula was used for the antioxidant activity determination.
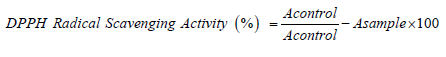
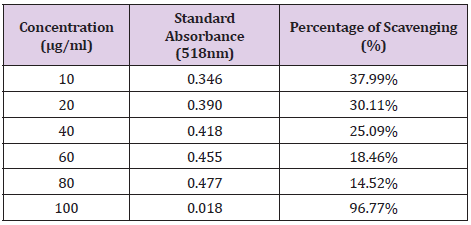
The concentration and absorbance of Gallic acid determined by U.V and concentration of MR ethanolic absorbance is shown in Table 8 and Graph 1 is drawn, respectively. The concentration, absorbance and the corresponding percentage of scavenging of BHT for DPPH Free Radical Scavenging Assay is shown in Table 9. The concentration, absorbance and the corresponding percentage of scavenging of ethanolic MR extract is placed Table 10 while Graph of percentage scavenging against the standard concentration is drawn in Graph 2. Table 11 represents The concentration, absorbance and the corresponding percentage of scavenging of ethanolic MR extract. The Graph of percentage scavenging against the sample concentration is shown in Graph 3.
Table 10: The concentration, absorbance and the corresponding percentage of scavenging of BHT (butylated hydroxytoluene).
Table 10: The concentration, absorbance and the corresponding percentage of scavenging of ethanolic MR extract.
Antihyperglycaemic Activity Screening of Maceration Extract
Animal
Healthy, adult, Sprague-Dawley (SD) rats (180 ± 20 g) were used for anti-hyperglycemic studies. The animals were obtained from Central animal house, AIMST University, Malaysia. Approval from the AIMST University Human and Animals Ethics Committee was obtained.
Induction of Diabetes
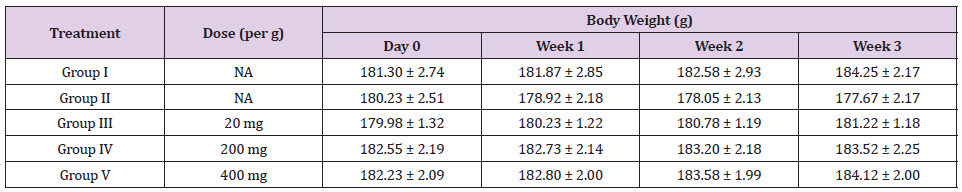
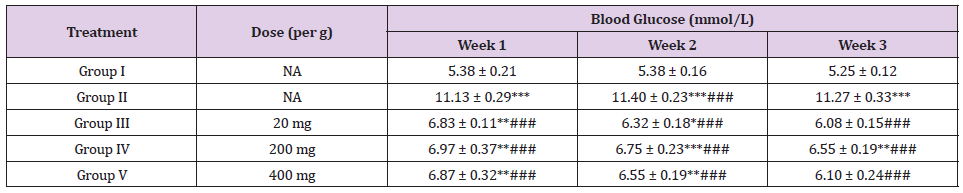
Healthy, adult male SD rats were used for the experiment. Diabetes mellitus will be induced in overnight fasted rats by administration of single intraperitoneal (I.P.) injection of freshly prepared streptozotocin (STZ) with a dose of 60 mg/kg/mL [12]. Anti-hyperglycaemic activity of ethanolic extract of MR was studied using STZ-induced diabetic rats. The diabetic animals were showed decreases in body weight but the results were not significant (Table 2). The diabetic rats showed significant increases in the levels of glucose throughout the study when compared with that of control animals. Whereas the diabetic animals treated with glibenclamide or ethanolic extract of MR (100/ 200 mg/kg) showed significant decreases in the glucose levels when compare with that of diabetic control animals. As shown in Tables 12 & 13, the normalized blood glucose levels of 200 and 400 mg/kg Morus rubra treated groups did not exhibit significant difference as compared to the 20 mg/ kg glibenclamide treated group since week one. This revealed that the anti-hyperglycaemic effect of the Morus rubra was comparable with that of glibenclamide.
Table 12: Effect of Ethanolic Maceration Extract of Morus rubra on Body Weight.
Note: NA: Not applicable. All the values are expressed as mean ± SEM (n= 6).
Table 13: Effect of Ethanolic Maceration Extract of Morus rubra on Blood Glucose.
Note: NA: Not applicable. All the values are expressed as mean ± SEM (n= 6).
*P < 0.01, **P < 0.01, ***P < 0.001 compare with that of control. ###P < 0.001 compared with diabetic control.
Discussion
One of the objectives of this research was to determine the phytochemical properties of MR. The MR extracted with 95% ethanol and the plant extract was undergone phytochemical screening. The presence of phytochemicals is a marker that the plant can be an essential source of precursors in the formation of newer synthetic drugs. In the present study, the results obtained for the phytochemical screening of MR ethanolic extract was analysed and it was found out that alkaloids, anthraquinone, carbohydrate and flavonoids were present in the sample. Moreover, the extractive value estimation should be carried out to determine the amount of the active constituents in a given amount of plant material and identify which extraction solvent is more suitable for the given plant sample. By using this method, phytochemicals present in the plant were extracted maximally. Phenolic compounds are very essential plant constituents which responsible for the antioxidant activity due to its redox properties. As a basis, Folin-Ciocalteu reagent was used to measure the phenolic content of different concentrations of plant extract. In this test, the Gallic acid was used as a standard and the total phenolic content was calculated by using the formula C = (A/B) x dilution factor. The results were derived from the calibration curve of Gallic acid, it was expressed as Gallic acid equivalents (GAE) per gram of samples. In the present study, the total phenolic content found was 6.420 mg GAE/g and 6.097 mg GAE/g for the concentrations of 50 and 200μg/ml respectively.
In this research work, the antioxidant potential of the MR extract was determined by using DPPH free radicals scavenging method and BHT was used as a standard. The DPPH is a stable freeradical which has a colour of purple, as the antioxidants react with the DPPH, the DPPH will be converted into non-radical DPPH-H form. Besides that, the colour of the DPPH will be decolorized from purple to light yellow colour. The degree of decolorization indicates the potency of the plant extracts in scavenging the free radicals. According to the results obtained, the IC50 was calculated for the BHT. The antibacterial activity of MR was tested by using the Escherichia coli, Staphylococcus aureus, Salmonella typhi, and Bacillus subtilis. The extract concentrations used in the agar well diffusion method were 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml, other than that, 5mg/ml of penicillin and ciprofloxacin was playing the role as a standard in this study. In the present study, there was no zone of inhibition observed in all concentrations of ethanolic MR extract. The microorganism used were all in active condition, this can be proven as the ciprofloxacin as well as penicillin showed a clear zone of inhibition. In addition, the bacteria strains utilized were susceptible towards MR as stated [15-20], therefore it was concluded that the extracts have no antibacterial activity at the concentration of 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml against gram negative. In the case of MIC test, the plant extract with the concentration of 0.1, 0.2, 0.4, 0.6, 0.8 and 1.0 mg/ml were utilized.
Conclusion
In conclusion, the phytochemical screening of ethanolic extracts
of MR had determined the presence of alkaloids, flavonoids,
carbohydrates and anthraquinones. The antibacterial activity of
MR was determined by using the agar well diffusion and MIC assay.
The micro-organism used were Escherichia coli, Staphylococcus
aureus, Salmonella typhi, and Bacillus subtilis. There was no
antibacterial activity observed in the agar well diffusion with
Escherichia coli and the MIC assay [21-29]. The antioxidant activity
of the plant extract was assessed through the method of DPPH test
while the total amount of phenolic compounds in the extract can
be determined through the TPC test. It was suggested that MR can
be a potential source as a natural significant antioxidant and less
marked antimicrobial agent. The MR is a natural source that worth
for further explore as it is inexpensive, natural, harmless and easy
to obtain.
For more Articles on : https://biomedres01.blogspot.com/








No comments:
Post a Comment
Note: Only a member of this blog may post a comment.