Pregnancy Induced Hypertension in Kabo Local Government Area of Kano State, Nigeria
Introduction
Despite the high technological inclination in the 21st century,
especially in the area of health, the rates of maternal mortalities and
morbidities are still very high in women worldwide. The occurrence
of maternal hypertensive disorders is found to have about 20.7
million women in 2013 and about 10% of pregnancies globally
are complicated resulting from pregnancy induced hypertension
Sharma, et al. [1]. In the United States, hypertensive disease of
pregnancy affects about 8% to 13% of pregnancies Mohan, et al. [2].
Annually, an estimated 2.9 million babies die during the neonatal
period and 2.6 million babies are stillborn around the world due to
PIH. According to WHO (2018), the rate of stillbirth is 21.9 per 1000
births in women with a pregnancy induced hypertension (PIH) and normotensive women 8.4 per 1000 live births in china Xiong T, et
al. [3]. Pregnancy Induced Hypertensions (PIHs) are responsible
for 70,000 maternal deaths universally, killing one woman every 11
minutes Magee, et al. [4]. It is the second leading cause of maternal
mortality in Bangladesh, according to the Bangladesh Maternal
Mortality Survey (2017), about 24 percent of the country’s maternal
deaths are caused by pre-eclampsia/eclampsia (PE/E) NIPORT, et
al. [5], which affects women during pregnancy, childbirth, as well as
postpartum. Factors, such as lack of health care provider capacities
to detect, prevent, and manage PE/E, late referrals of HIP clients,
late attendance and lack of antenatal care (ANC) and awareness
about PE/E among communities have been associated as reasons
for most of these preventable deaths Warren, et al. [6].
Deruelle, et al. [7] reported that about 25 percent of women
with PIH, especially those with a dangerous condition, experience a
decline of end-organ functions during puerperium (the 6 to 8 weeks
after delivery, during which pregnancy changes return to baseline).
PE early in pregnancy (less 34 weeks of gestation), presenting in
a severe form, or persistence of proteinuria more than three to
six months after delivery suggests possible chronic hypertension
or renal disease. Women with pre-eclampsia are also at increased
risk for venous thromboembolism in the postnatal period (after
delivery), and those women should receive thromboembolic
prophylaxis after delivery until they are fully recovered, usually
within four to six weeks RCOG [8]. Similarly, women with preterm
pre-eclampsia and gestational hypertension have been found
to develop persistent cardiovascular impairment one year after
delivery Melchiorre, et al. [9], including other chronic diseases such
as chronic hypertension, stroke, renal disease, diabetes mellitus,
and ischemic heart disease. Infants born to women with PIH also
require special attention in the immediate postnatal period due to
a combination of short and long term risks. Standard international
guidelines recommend lifelong care and monitoring, or a minimum
of care and monitoring for six months to one year after delivery.
Studies propose that complications associated with PIH continue in
the immediate postnatal period and longer NICE [10].
One of the goals of the United Nations Sustainable Development
Goal (SDG) is to reduce the global maternal mortality ratio to less
than 70 per 100,000 live births by 2030 United Nations [11]. The
SDGs aim to uphold the momentum of the Millennium Development
Goals (MDGs), which in its relentless effort catalyzed a global
reduction in maternal deaths from approximately 390,000 in 1990
to 275,000 in 2015 Graham, et al. [12] United Nations, 2018). The
strains of maternal mortality remain unduly borne by women in
less-developed countries, particularly in sub-Saharan Africa (66%,
201,000 deaths) and southern Asia (22%, 66,000 deaths). One of
the leading causes of maternal death (and disability) worldwide is
pregnancy induced hypertension Payne, et al. [13]. The statistical
figures of this problem in less developed countries have varied from
4.0% to 12.3% (Sebastia et al., 2015; Berhe et al., 2018). Pregnancy
induced hypertension is also one of the major leading causes of
pregnancy associated with morbidity and it is the most recurrent
cited cause of maternal death Ethiopian Journal of Health Science
[14]. Despite the fact that hypertensive conditions in pregnancy
is leading causes of maternal morbidity and mortality during
pregnancy, little is known about its magnitude among pregnant
women in Kano and, specifically in Kabo Local Government Area.
This study therefore aims to fill this gap by considering pregnancyinduced
hypertension, the signs, associated risk factors and
prevention and management among pregnant woman attending
antenatal service in Kabo.
Basic Tools of Scientific Inquiry
The study was guided by the following research questions:
1. What are the signs of pregnancy induced hypertension among
women in Kabo Local Government Area of Kano State?
2. What are the risk factors associated with pregnancy induced
hypertension among women in Kabo Local Government Area
of Kano State?
3. What are the preventions measures of pregnancy induced
hypertension in Kabo Local Government Area of Kano State?
Literature Review
Pregnancy Induced Hypertension (PIH) known as toxemia or
preeclampsia is a form of high Blood Pressure (BP) in pregnancy.
PIH is one developing after 20 weeks of gestation without other
signs of preeclampsia. It is a known cause of premature delivery,
intrauterine growth restriction (IUGR), placental abruption and
fetal death, as well as maternal mortality and morbidity (Gombe
et al., 2011). It is characterized by either blood pressure levels
of 140/90 mm Hg or higher after 20 weeks of gestation, or a
blood pressure rise greater than 30/15mmHg from early or prepregnancy
baseline or a rise of mean arterial pressure of more
than 105 mmHg. This due to the development of arterial high
pressure in a pregnant mother after 20 weeks of gestation, which
may or may not have protein in urine and has a blood pressure
of or more than 140/90 mmHg (National Guidelines for Quality
Obstetrics, 2004). Of all the pregnancy related complications in the
world, pre-eclampsia and eclampsia present 10% major causes of
maternal and prenatal morbidity and mortality, with pre-eclampsia
affecting 5-7 % of all pregnancies, Srinivas, et al. [15]. Hypertensive
disorders during pregnancy is among the leading cause of maternal
and fetal mortality in obstetric practice that can prevent the baby
from getting enough blood and oxygen harming their liver, kidney,
brain, and heart, causing end organ damage, Palacios, et al. [16].
Pregnancy induced hypertension is a major cause of maternal
morbidity and mortality in the United States. There is an
approximately one maternal death due to preeclampsia-eclampsia
per 100,000 live births, with a case-fatality rate of 6.4 deaths per
10,000 cases Livington, et al. [17]. The outcome of hypertension in
pregnancy is affected by multiple factors. These include gestational
age at onset, severity of disease, and the presence of comorbidities
like diabetes mellitus, renal disease, thrombophilia, or pre-existing
hypertension Heard, et al. [18]. Similarly, a study conducted in
Latin America and Caribbean, Pakistan, New York, and Sri Lanka
identified null parity, multiple pregnancies, history of chronic
hypertension, gestational diabetes, fetal malformation and obesity
as the risk factors for developing pregnancy induced hypertension
Dolea [19]. Furthermore, life-threatening maternal age (less than
20 or over 40 years), history of PIH in previous pregnancies, preexisting
diseases like renal disease, diabetes mellitus, cardiac
disease, unrecognized chronic hypertension, positive family history
of PIH, which shows genetic susceptibility, psychological stress,
alcohol use, rheumatic arthritis, very underweight and overweight,
and low level of socioeconomic status are the risk factors for PIH
Abeysena, et al. [20].
One important aspect of diagnosing and managing hypertension
in pregnancy is presiding out secondary causes. These causes can
add to both the maternal, fetal morbidity and mortality. Records
from the Nationwide Inpatient Sample (NIS) of hospitalizations for
delivery between 1995 and 2008 showed that out the patients with
chronic hypertension (1.15% of the sampled population), 11.2%
had secondary causes. Secondary hypertension had higher odds of
adverse maternal and fetal outcomes when compared to essential
hypertension (odds ratio (OR), 11.92 vs 10.18 for preeclampsia,
51.07 vs 13.14 for acute renal failure, 4.36 vs 2.89 for spontaneous
delivery < 37 weeks) Bateman, et al. [21]. Examples of secondary
forms of hypertension are chronic kidney disease (most common
cause), hyperaldosteronism, Reno vascular disease, obstructive
sleep apnea, Cushing’s syndrome, pheochromocytoma, thyroid
disease, rheumatologic diseases (e.g. scleroderma or mixed
connective tissue disease), and coarctation of the aorta; lack of
understanding on how to diagnose and treat these conditions
during pregnancy may lead to a higher morbidity and mortality
Malha [22].
Pregnancy Induced Hypertension in Nigeria
In Nigeria, an incidence of 20.8% of pregnancy induced
hypertension had been reported in a study of pregnant women
attending antenatal clinics in a Teaching Hospital in South-South
Ebeigbe, et al. [23]. Similarly, prevalence rates of hypertensive
conditions of pregnancy range from 17% to 34.1% Singh, et al.
[24]. In 2009, the occurrence of PIH ranges between 2% to 16.7%
Abubakar, et al. [25]. In 2011, Enugu town had 3.3% per 77 cases of
PIH out of 2337 cases Ugwu, et al. [26]. In 2014, according to Singh,
et al. [27], the prevalence of hypertensive disorders was estimated
to be higher than 17% in Nigeria. Akeju, et al. [28] suggested that
women have health seeking behaviors, which range from buying
over the counter drugs to relieve headache, consulting families
on what to do with odema, epigastric pain and blurred vision,
consulting a spiritual or traditional healer on convulsing and coming
to hospital. All these health-seeking behaviors may delay coming to
hospital, worsening the PIH complications. Between the periods of
1990 and 2015, 10.7 million maternal deaths were stated globally,
in spite of the fact that maternal mortality ratio had fallen by 44%
over these periods. WHO [29]. Out of this total number, developing
countries accounts for about 99% of the global deaths in 2015, with
Sub-Saharan Africa accounting for bumpily 66%.
Study by WHO [29] showed that Nigeria and India are estimated
to account for over one third of all maternal deaths globally in 2015,
contributing 19% and 15% respectively. Furthermore, the study
also revealed that in the West African sub-region, Nigeria with a
maternal mortality ratio (MMR) of 814 ranks second, after Sierra
Leone 1360 MMR. With this MMR, Nigeria could not meet the
MDG5A target in 2015, which aims to reduce maternal mortality
ratio by 75% of its 1990 level by 2015.Among the causes of maternal
mortality, hypertension ranks second (14%) after hemorrhage Say,
et al. [30]. In Nigeria, hypertensive disorders of pregnancy could
be a contributory factor to the rising prevalence of hypertension,
which has been predicted to escalate up to 39.1 million by 2030, if
the current inclination in figures continues Adeloye, et al. [31].
Empirical Review
Studies conducted by Butalia, et al. [32] and Regitz Zagrosek,
et al. [33] revealed that there remain terminology and definition
disagreements across international guidelines for hypertension.
Hypertension itself has been defined over the years by diastolic
or systolic readings alone, as well as by changes in pressures
throughout pregnancy Chappell, et al. [34]. Similarly, limits for what
is considered severe hypertension have been different. Semantics
have clinical implications, and systematic reviews often have
to compare studies or populations, which are assumed to be the
same, rather than standardized Abalos, et al. [35]. Therefore, the
International Society of the Study of Hypertension in Pregnancy
(ISSHP) identified this as one of the factors for the range of
controversies surrounding the treatment of hypertension during
pregnancy and appointed a committee to address them beginning
in 1998 Brown, et al. [36]. Moreover, studying several international
guidelines, definitions are more standardized; however, there are
still disagreements in sphygmomanometer intervals that define
hypertension, precise definitions of proteinuria, the terms used
to characterize blood pressure in the non-severe range, and even
terminology used to classify the hypertensive disorders themselves
Redman [37,32,33]. All of these reflect that the understanding of
hypertensive disorders of pregnancy remains unsolidified and
further research is necessary before a universal unanimity is
reached on how to treat these disorders.
Hypertensive Disorder of Pregnancy (HDP) is defined as high
blood pressure during pregnancy, is one of the direct causes of
maternal and child mortality AOM [38]. It is measured by blood
pressure level greater than 140/90 mm Hg after 20 weeks of
gestation. Austere forms of HDP are reflected through blood
pressure intensities of 160/100 mm Hg and more NHLBI [39].
Furthermore, studies by Magee, et al. [40] revealed that HDP are
responsible for 70,000 maternal deaths globally, killing one woman
every 11 minutes. HDP is the second leading cause of maternal
mortality in Bangladesh, according to the Bangladesh Maternal
Mortality Survey 2017, with approximately 24 percent of the
country’s maternal deaths caused by pre-eclampsia/eclampsia
(PE/E) NIPORT [5], which affects women during pregnancy,
childbirth, as well as postpartum. Factors, such as lack of health
care provider capacities to detect, prevent, and manage PE/E, late
referrals of HDP clients, late attendance and lack of antenatal care
(ANC) and awareness about preeclampsia or eclampsia among
communities have been associated as reasons for most of these
preventable deaths Warren et al. [6].
Theoretical Framework
This study is anchored on two theories, which include: the
Theory of Reasoned Action (TRA) and the Theory of Planned
Behavior (TBP). Theory of Reasoned Action was formulated by
Martin Fishbein and IcekAjzen towards the end of the 1960s. On the
other hand, IcerkAjzen proposed the Theory of Planned Behaviour
in 1985; which was an extension from the TRA. The Theory of
Reasoned Action and Theory of Behaviour Planned combine two
sets of belief variables, which are ‘behavioural attitudes’ and
‘the subjective norms’. The behavioural attitudes are defined
as the multiplicative sum of the individual’s relevant likelihood
and evaluation related to behavioural beliefs. On the other hand,
subjective norms are referent beliefs about what behaviors others
expect and the degree to which the individual wants to comply with
others’ expectations. The summary of the two theories suggest
that a person’s health behavior is determined by their intention
to perform a behavior (behavioural intention) is predicated by a
person’s attitude toward the behavior, and the subjective norms
regarding the behavior. The Theory of Reasoned Action has been
criticized because it is said to ignore the social nature of human
action Kippax [41]. These behavioural and normative beliefs are
derived from individuals’ perceptions of the social world they
inhabit, and are hence likely to reflect the ways in which economic
or other external factors shape behavioural choices or decisions.
In addition, there is a compelling logical case to the effect that
the model is inherently biased towards individualistic, rationalistic,
interpretations of human behavior. Its focus on subjective
perception does not essentially permit it to take meaningful
account of social realities. Individuals’ beliefs about such issues
are unlikely going to reflect the accurate potential and observable
social facts. As such, the Theory of Planned Behaviour updated the
Theory of Reasoned Action to include a component of perceived
behavioural control, which brings about one’s perceived ability to
enact the target behavior. Actually, perceived behavioural control
was added to the model to extend its applicability beyond purely
volitional behaviours. Previous to this addition, the model was
relatively unsuccessful at predicting behaviours that were not
mainly under volitional control. Therefore, the Theory of Planned
Behaviour proposed that the primary determinants of behaviour
are an individual’s Behavioural intention and perceived behavioural
control. A constructive use of the TRA and TBP in research and
public health intervention programmers might well contribute
valuably to understanding issues related to health inequalities and
the roles that other environmental factors have in determining
health behaviours and outcomes. In spite of the criticism, the
general theoretical framework of the TRA and TPB have been
widely used in the retrospective analysis of health behaviours
and to a lesser extent in predictive investigations and the design
of health interventions Hardema, et al. [42]. This is why there is a
connection between the study and the theory, since the tenets of
the theories are located within the pore of the study.
Methodology
The study was conducted in Kabo Local Government Area of Kano State, Nigeria. It has an area of 341km2 and a population of 153,828 NPC [43]. The study comprises of women within the reproductive ages of 14-45, pregnant and married in Kabo Local Government Area of Kano State. Women were purposively selected for the study not just because of their ability to conceive but they are the ones that do encounter pregnancy induce hypertension. Two nurses were selected from the Cottage Hospital in the local government based on their long working experiences and competencies in the facility. This makes a total sample of twenty one (22) respondents. The study used Interpretative Phenomenological Analysis (IPA). Purposive sampling method was used in selecting the respondents for in-depth interview. Kabo Cottage Hospital was purposively selected, which is bigger compare to other two in the local government with an average of 52Anti-natal Care Attendance (ANC) and 36 live births in the facility monthly. The 22 respondents were interviewed by structural interview method, using tape recorder, note book and biro as data gathering instruments. The respondents were tag with codes like respondent 1, 2, 3, 4 and 5, etc. Based on the in-depth interview method, the data was presented using interpretative analysis.
Findings and Discussion
In attempt to mention the signs of pregnancy induced
hypertension, respondent 1 states that: “signs of pregnancy induced
hypertension are many, however, she stated the following as part
of the signs as follows: chest pain and headache”. Corroborating,
respondent 2 put forward the followings as some of the signs:
“blurred vision and dizziness,” while respondents 3, 4, 5 and 6
agreed that pedal oedema and epitaxies are also among the signs.
Similarly, a study by Haque [44] stated that high blood pressure,
headache, blurred vision, swelling in extremities, nausea or
vomiting, fatigue and sudden weight gain are among the signs of
pregnancy induced hypertension. Furthermore, Magee, et al. [45]
noted that the patient with severe PIH should be evaluated for signs
of preeclampsia, as generalized edema, including that of the face
and hands, rapid weight gain, blurred vision or scotomata (ie, areas
of diminished vision in the visual field), throbbing or pounding
headaches, epigastric or pain associated with upper quadrant,
oliguria (urinary output < 500 mL/d),nausea with or without
vomiting, hyperactive reflexes, chest pain, tightness and shortness
of breath. Blood pressure should be measured and recorded at
every prenatal visit, using the correct-sized cuff, with the patient
in a seated position. Leeman [46] said gestational hypertension
is a clinical diagnosis confirmed and established by at least two
accurate blood pressure tests in the same arm in women without
proteinuria, with readings of ≥ 140 mm Hg systolic and ≥ 90 mm
Hg diastolic.
It should then be determined whether the patient’s
hypertension is mild or severe (i.e. blood pressure > 160/110 mm
Hg). When asked about the risk factors associated with pregnancy
induced hypertension, respondent 1 states that: “parts of the
risk factors associated with pregnancy induced hypertension are
indefinitely large numerically, nonetheless, she listed the followings
as part of the risk factors: “multiple gestations, elderly prim gravida
and high parity.” Moreover, respondent 2 stated “polyhydramnios
and essential hypertension,” while respondent 3 stated “kidney
disease.” All the respondents agreed that high salt intake (in diet),
obesity and stress also contribute to the risk factors associated
with pregnancy induced hypertension. Bansode [47] also found
that some of the factors associated with pregnancy induced
hypertension include first pregnancy, new partner/paternity, age
<18 years or >35years, black race, obesity (Body Mass Index, BMI
≥ 30), inter-pregnancy interval <2 years or > 10 years and use of
selective serotonin reuptake inhibitors (SSRIs) beyond the first
trimester; while placental or fetal risk factors include multiple
gestation, hydropsfetalis, gestational trophoblastic disease and
triploidy. Similarly, Umegbolu [48] reported that the overall
incidence of PIH among pregnant women in Enugu State, Southeast
Nigeria (2006-2015) was found to be 5.9%.
The study identified annual variations in the incidence of PIH
(rising and falling trends between 2006 and 2015) among the
pregnant women. The incidence of PIH was highest among those
women above 35 years (13.5%), compared to those whose age is
less than 20 years (9.1%) and those between 20-35 years (5.1%).
The occurrence was also higher in the nulliparous (prim gravidae)
(7.7%) compared to the multiparous ones (5.5%). Furthermore,
Anujeet, et al. [49] stated that hypertension, collagen vascular
disease, obesity, black race, insulin resistance, diabetes mellitus,
gestational diabetes, increased serum testosterone concentrations
and thrombophilia, clotting disorders, and hemolysis, elevated
liver enzymes, low platelet count (HELLP) syndrome are also
responsible risk factors for PIH. Similarly, age and parity are two
of the identified maternal risk factors for the development of
pregnancy induced hypertension. Extreme ages (age below 20
years and above 35 years) are known to be associated with higher
incidence of pregnancy induced hypertension. Like the overall
incidence of pregnancy induced hypertension, incidence among
various age groups and parity varies from place to place. In Karachi,
Rehman, et al. [50] found an incidence of 9% among older women
and 27% among prim gravidae. However, Sajith, et al. [51] reported
an incidence of pregnancy induced hypertension of 41.3% among
18-22 years old patients in their study.
Furthermore, Ahmed, et al. [52] states that educational
attainment of women is also a factor that contributes to developing
pregnancy induced hypertension. This is because illiterate mothers
are more likely to suffer from hypertension during pregnancy
than their counter parts. There is every tendency that educated
mothers are likely to be aware of pregnancy related complications
and its consequences, marry educated husband that facilitate
couples discussion on maternal health care utilization, likely
to be autonomous in decision making and hence meeting her
reproductive needs. Generally, education increases health seeking
behaviours of women. The study of Swati, et al. (2014) stated being
unmarried as a risk factor and prognosticator of PIH, which is an
exceptional result from this study. The study further explains that
although marital status is hardly reported as a risk factor in the
literature, a possible explanation in a resource poor country like
Nigeria could be due to worry about the financial burden of single
parenting in environment, which lacks any form of child welfare
support. Moreover the stigma of having a child outside marriage is
a potential source of concern and anxiety. In addition, Zusterzeel,
et al. [53] had emphasized immunological intercourse as the
prevention of this maternal-fetal conflict called pregnancy induced
hypertension. There is an association of pregnancy-induced
hypertension with duration of sexual co-habitation before the first
conception. Male ejaculation is said to protect a woman if she has
been repeatedly been exposed to it.
Regarding the prevention and management of pregnancy
induced hypertension, respondent 10 states that “regular antenatal
care and regular check-up/ BP checking are sine qua non.”
Corroborating, all the respondents agreed that low salt intake,
avoiding alcohol, regular intake of water drug therapy for severe
hypertension, treatment of underlying medical conditions like
kidney problem and avoidance of strenuous exercises or stress are
parts of the prevention and management. The findings of the studies
are similar with Singh [54] that PIH can be prevented and managed
by low salt intake, drinking at least eight glasses of water a day,
regular BP check-up, increase the amount of protein-rich foods and
decrease the amount of fried and junk foods, regular exercise and
enough rest, elevate your feet several times during the day, avoid
drinking alcohol and beverages containing caffeine, prescription
of drug therapy and additional supplements by a medical doctor.
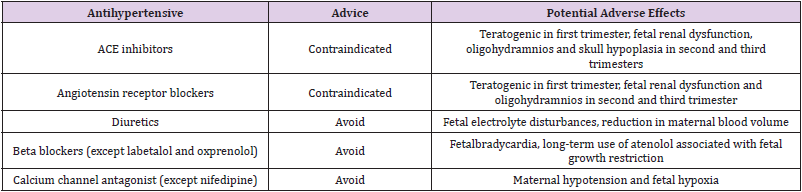
In drug management of pregnancy induced hypertension, ACOG
[55] states that labetalol and extended-release nifedipine are
first line drugs as they are safe and effective. Similarly, Morgan, et
al. [56] emphasized, although beta-blockers other than labetalol
are less well investigated, if labetalol cannot be used metoprolol,
propranolol, pindolol and acebutolol may be considered. Extendedrelease
nifedipine is the most widely used calcium channel blocker.
Amlodipine has been used, but the data are limited. Inhibitors
of the Renin Aangiotensin Aaldosterone System (RAAS) are totally
contraindicated. This includes angiotensin converting enzyme
(ACE) inhibitors, angiotensin receptor blockers (ARBs) and direct
renin inhibitors. The package insert of these agents comes with a
black box warning stating that usage during the second and third
trimesters can cause injury and death to the developing fetus;
therefore, discontinuation is indicated as soon as pregnancy is
detected. RAAS inhibitors use in the second and third trimesters
is associated with renal agenesis, pulmonary hypoplasia and
intrauterine growth retardation, IUGR. The proof of fetal injury
during first trimester exposure is unclear. Post-delivery lactating
mothers may use enalapril, captopril or quinapril because they have
low concentration in breast milk; labetalol may be continued only in
lactating mothers Colaceci [57]. In addition, Donovan [58] says the
choice of antihypertensive drugs to be used depends on whether
breastfeeding is tried or attempted. When the woman desires to
breastfeed, deliberation must be given to potential transfer of the
drug into breast milk. This is due to the established evidence that
most drugs safely used in pregnancy are excreted in low amounts
into breast milk and are compatible with breastfeeding. (Table 1)
shows antihypertensive drugs of those to use and those to avoid
during lactation.
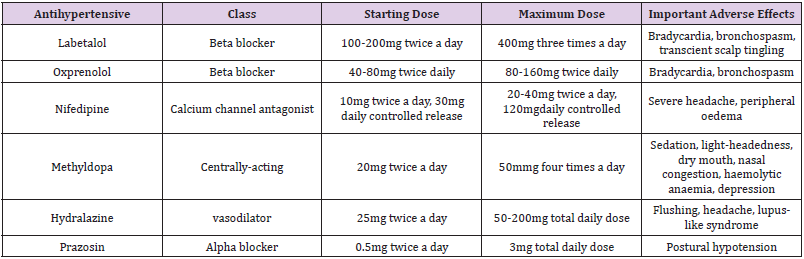
In an instance when there is no desire to breastfeed and adequate contraception is used, the choice of antihypertensive drug is the same as for any other non-pregnant woman or patient. While (Table 2) shows the antihypertensive drugs used in pre-eclampsia, which are the same as those used to treat chronic and PIH Lowe et al. [59-61].
Conclusion
The strains of maternal mortality remain unduly borne by
women in less-developed countries, particularly in sub-Saharan
Africa. Between the periods of 1990 and 2015, 10.7 million
maternal deaths were stated globally, in spite of the fact that
maternal mortality ratio had fallen by 44% over these periods. Out
of these total numbers, developing countries account for about 99%
of the global deaths in 2015, with Sub-Saharan Africa accounting
for bumpily 66% with 201,000 deaths. PIHs are responsible for
70,000 maternal deaths universally, killing one woman every 11
minutes. Nigeria, in 2014, recorded the prevalence of pregnancy
induced hypertension and was estimated to be about 20.8%
among pregnant women attending antenatal clinics in Teaching
Hospitals in South-South. Based on these problems, the findings of
this study attest that the signs of pregnancy induced hypertension
in Kabo Local Government include chest pain, headache, blurred
vision, dizziness, pedal oedema and epitaxies. Finally, the risk
factors are multiple gestations, elderly prim gravida, high parity,
polyhydramnios, essential hypertension, kidney disease, high salt
intake (in diet), obesity and stress.
For more Articles on : https://biomedres01.blogspot.com/

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.