Keratoacanthoma and Well Differentiated Squamous Cell Carcinoma Have a Distinct Prognosis Running Head: Prognosis of Keratoacanthoma
Introduction
Keratoacanthoma (KA) is a rapidly growing skin tumor thought to originate from the hair follicle [1]. The exact classification of the tumor is still a matter of debate. Due to its ability to spontaneously regress some consider KA a benign lesion [2]. However, KA can also display perineural as well as venous invasion [3,4] and cases of metastatic KA were also reported [5,6] suggesting the classification as a subtype of well differentiated SCC is more appropriate. On a molecular level, the etiology of KA and Squamous Cell Carcinoma (SCC) seems to differ, since deletion of polarity proteins in mouse models can rescue SCC formation but promote KA formation [7]. Expression of tumor suppressors and promoters is also different in KA compared to SCC with a gain of 11q and subsequent amplification of the cyclin D1 locus being the most frequent aberration in KA [8]. Mutated p53 is more frequently found in SCC compared to KA and the cell cycle inhibitor p16 is expressed in KA but downregulated in SCC [9]. Additionally, the tumor microenvironment of KA and SCC is different providing a possible explanation for the ability of KA to regress [10].
The therapy of KA usually consists of complete excision since it is impossible to predict whether the tumor will regress or progress to invade the underlying tissue and even metastasize [5,6]. Since current guidelines on SCC do not take the KA histological subtype as a prognostic factor into account the question whether KA has a distinct prognosis compared SCC remains open. This question is particularly relevant since KA often have an increased tumor thickness due to rapid growth and may unnecessarily fall into the category of high risk SCC thereby burdening the financial system with frequent follow-up of these patients. In this study, we compared the prognosis of KA with that of well differentiated SCC without KA histology (wSCC) and found that KA histology favorably impacts metastasis-free survival but not local relapse-free survival.
Patients and Methods
A retrospective analysis of medical records of the Department of Dermatology, University Hospital of Cologne identified 403 KA and 905 wSCC. Tumors with an incomplete excision were excluded. The histopathologic criteria for KA were a crateriform tumor surrounding a central keratin plug showing epithelial lipping [1,11]. Solar elastosis was defined as a fibrillary basophilic material in the upper dermis [12]. Immunosuppression was defined as use of chemotherapy, other malignancies than non-melanoma skin cancer and use of immunosuppressive drugs. Diabetes was not considered immunosuppressive. Statistical evaluations were performed with the statistical software package IBM SPSS, version 20.0. Graphs were made using GraphPad Prism, version 5.0. Student’s t test was used with continuous variables, while the X2 test was used for categorical variables. Survival rates were calculated by the Kaplan– Meier method and compared using log-rank tests. A P-value of <0.05 was considered significant.
Results
A total of 403 KA and 905 wSCC were retrospectively analyzed. The median follow-up was 23 months. 8 Patients with KA (2%) developed metastasis after a median of 7 months (range 1-49) and 35 patients with wSCC (3.9%) developed metastasis after a median 8 of months (range 1-37). Except for 2 patients with wSCC, who developed lung metastasis all patients developed metastasis of skin and/or lymph nodes. The metastasis-free survival was significantly decreased in the wSCC groups compared to the KA group (p=0.042) (Figure 1). 9 KA (2.2%) recurred locally following complete excision after a median of 32 months, while after a median of 8 months 24 wSCC (2.7%) recurred locally. The local recurrence-free survival was not significantly different in the KA group compared to the wSCC group (p=0.426) (Figure 1).
Figure 1:
a) Metastasis-free survival in keratoacanthoma compared to well differentiated squamous cell carcinoma (SCC) (p=0.042).
b) Local recurrence-free survival in keratoacanthoma compared to well differentiated SCC (p=0.436).
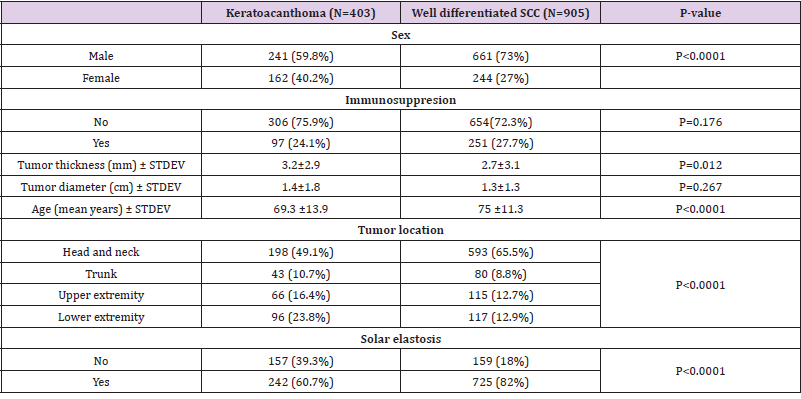
Patients with KA were more likely to be female (40.2% versus 27%, p<0.0001) and to have thicker lesions compared to patients with wSCC (tumor thickness 3.2 vs 2.7, p=0.012) (Table 1). Age was also significantly decreased in KA compared to wSCC (p<0.0001). Furthermore the patterns of tumor localization were different in KA compared to wSCC, with KA occurring more frequently on the lower extremity and to a lesser extent in the head and neck region. Since the difference in tumor location could be explained by sun exposure, we analyzed the presence of solar elastosis in the periphery of tumors and found that solar elastosis occurred more frequently in wSCC compared to KA (82% vs 60.7%, p<0.0001). In contrast, the presence of immunosuppression and tumor diameter was not significantly different in the KA group compared to the wSCC group (Table 1).
Table 1: Clinical data keratoacanthoma compared to well differentiated SCC.
Note: SCC- squamous cell carcinoma; STDEV-standard deviation.
Discussion
Currently, no specific guideline for the follow-up of patients
with KA exists and it is unclear whether the guidelines regarding
the follow-up of SCC [13] can also be applied to KA. To our
knowledge, this the first study comparing the prognosis of KA with
wSCC. We found that the metastasis-free survival was significantly
increased in KA compared to wSCC. 2% of KA developed metastasis
in the skin and regional lymph node indicating that similar to
SCC ultrasound of the regional lymph nodes is also important in
patients with KA. Since local recurrence-free survival was not
different in KA compared to wSCC we propose to use the same
frequency of physical examinations of the skin in KA as in SCC [13].
In line with the hypothesis that SCC and KA have different etiologies
our study could show that clinical and pathologic characteristics
were also significantly different in the wSCC group compared
to KA. As expected from a rapidly growing tumor, the tumor
thickness of KA was significantly increased compared to wSCC.
Interestingly, patients with KA were more likely to be female and
were significantly younger compared to patients with wSCC further
underlining the different etiology of the two tumors.
In accordance with a previous study [14] we could show that
the predominant distribution of KA is on the lower extremity while
SCC were mostly localized in the head and neck region. Moreover,
the presence of solar elastosis was significantly increased in the
KA group suggesting sun exposure plays a more minor role in KA.
Consistent with the hypothesis that UV plays a less important role
in the etiology of KA the mutation burden of KA is lower compared
to cutaneous SCC [15]. Further supporting a different etiology of KA
and SCC the rate of HPV DNA detection was higher in KA compared
to wSCC suggesting a possible viral etiology in the pathogenesis of
KA [16]. This would then imply that immunosuppression would
play a more important role in the etiology of KA compared to SCC,
however the incidence of immunosuppression was not different
in KA compared to the wSCC group in our study, suggesting other
etiologic factors such as the tissue microenvironment could play a
role.
Conclusion
We could show that KA histology favorably impacts metastasisfree survival but does not influence local relapse-free survival.
For more
Articles on : https://biomedres01.blogspot.com/




No comments:
Post a Comment
Note: Only a member of this blog may post a comment.