Use of the Leaf-Aqueous Extract of Pseudopanax Arboreus (Araliaceae) (L.F. Phillipson) is Void of Toxic Effects
Introduction
Over the past two decades, there has been an increase in the
use of plant-based medicine worldwide. It is estimated that
threequarters
of the world’s population use herbal medicine for their
healthcare [1]. Interestingly, the World Health Organization (WHO)
has encouraged this alternative medicine for the prevention and
treatment of diseases and according to estimates put up by this organ
(WHO), more than 80% of the population in Africa uses the doctor’s
plants to meet their need for care and health [2]. The toxicity of
chemical products, the high cost of chemical drugs, the remoteness
and /or insufficiency of health centers, especially in rural settings,
which limits the genuine handling of public health problems, have
favored the use of such drugs [3]. As a result, traditional medicine
can be considered an integral part of primary healthcare and is
used to improve access to healthcare [4]. Unfortunately, because
plants are natural, they are considered to be non-dangerous, and
the population use them in many and very different contexts. The
products used are often the mixture of plants whose knowledge and
requirements of preparation and consumption are not controlled.
Thus, although plants may be effective in treating some ailments,
they may contain potent chemical compounds that cause adverse
effects and toxicity, especially when administered at high doses and
for a long period [5,6]. For instance, Peganum harmala is a plant
with scientifically proven analgesic, hypoglycemic, anti-nociceptic
and anti-parasitic properties, but whose prolonged administration
leads to hepatotoxic and nephrotoxic abnormalities. Also,
Astragalus hamosus is effective in the treatment of gastrointestinal
diseases, respiratory problems and headache, but causes liver
and kidney dysfunction at higher doses and following long term
administration [7].
It is therefore essential to ensure not only the clinical efficacy
and the quality, but especially the safety of any medicinal herbal
preparation before making it available to consumers. Toxicity tests
are therefore indispensable and accompany the biological activity
test in the course of the selection of new molecules [8]. For this, a
renewed interest has been brought to phytotherapy to deepen the
analysis of its therapeutic efficacy and especially its toxicity aspect.
P. arboreus is, belonging to the Family Araliaceae, is commonly used
in traditional medicine in the treatment of many ailments such as
hypertension, male infertility and male sexual dysfunction. It has
already been the subject of several scientific studies in which the sexenhancing
properties of the aqueous [9] and methanol [10] extracts
of its leaves have been demonstrated. In other investigations, its
potentials to reverse clinically induced male sexual dysfunction
(MSD) have been proven [11]. Further studies have evaluated the
time-response activity of the leaf-methanol extract of the plant
[10]. However, till date, no study has been conducted in relation to
its toxicological effect, hence the question of whether the aqueous
extract of P. arboreus would have toxic effects in the body. This
would suggest that in addition to its proven biological efficacy, the
aqueous extract of P. arboreus would be devoid of toxic effects. This
work therefore had as aim to evaluate the toxicological effect of
aqueous extract of P. arboreus in rats.
Materials and Methods
Plant Collection and Preparation of the Aqueous Extract
Fresh leaves of P. arboreus were collected from Ntenako village, Manyu Division, South-West Region of Cameroon, under the guide of a local tradi-practitioner who confirmed the plant’s identity. Its authentication, processing and production of the aqueous extract were done following the same procedures as outlined in our previous study [9].
Chemical Products or Reagents
Products or reagents used in this study included assay kits for triglycerides and total cholesterol (IVD DIALAB, Austria), Creatinine, AST (aspartate aminotransferase), ALP (alkaline phosphatase), ALT (alanine amino transferase) (Elabscience, USA) and Albumin (BioVision Inc, USA). All were purchased and stored under recommended conditions until used.
Breeding of Animals
Animals used were rats of the Wistar Strain of either sex that were bred in the Animal Facility of the Department of Zoology and Animal Physiology of the Faculty of Science, University of Buea under standard conditions of temperature, humidity and light (12H cycle). They were given free access to water and a standard laboratory diet.
Acute Toxicity
In order to assess the toxic nature of a compound, acute oral toxicity is the first step to be carried out [12]. Acute toxicity testing involves the determination of lethal dose, the single dose that kills 50% of the tested group of animals within 24 hours. Acute toxicity studies of the leaf aqueous extract of P.arboreus were carried out in male rats by using Organization for Economic Co-operation and Development (OECD) guidelines [13]. Before oral administration of a single dose of the test substances, the rats were deprived of food for 3 hours. They were randomly divided into 3 groups of 7 rats each. Animals of group 1 were administered 10 ml/kg distilled water to serve as the control; while those of groups 2 and 3 received 2000 and 5000mg/kg of the aqueous extract, respectively. All animals were observed for general behavioral changes (somnolence, convulsion, fatigue, increase heart rate); symptoms of toxicity and mortality after treatment for the first four (critical) hours, then over a period of 24 hours and thereafter, 2 hours daily for 14 days. Meanwhile, body weights were measured daily. Abnormal findings were recorded with the time of onset and disappearance. On the 14th day, all animals were sacrificed and selected organs (lung, liver, heart and kidney) isolated and processed for macroscopic observations [12].
Sub-Acute Toxicity
When treatment related toxicity is not identified in acute
toxicity, sub-acute toxicity is assessed to ensure safety after
repeated exposure over a relatively long period of time. Sub-acute
toxicity study can be used to determine the undesirable effects of
continuous or repeated exposure of part of an average life laboratory
animal to a plant extract and to provide information of target organ
toxicity. Like in the acute toxicity test, sub-acute toxicity study (28-
day repeated oral toxicity study) was also carried out according to
OECD 407 guidelines [14]. Eight (8) weeks old (110-120g) rats of
either sex were divided into 4 groups with 10 animals (5 males plus
5 females) in each group. Animals of group 1 received 10ml/kg of
distilled water and served as a control group whereas groups 2, 3
and 4 were given the aqueous extract at 250 mg/kg, 500 mg/kg and
1000 mg/kg body weight, respectively. All animals were observed
for 4 hours daily for mortality and morbidity till the completion of
the experiment. They were observed for clinical signs and the time
of onset, duration of these symptoms, if any were recorded. Body
weights of the rats in all groups were recorded once before the start
of treatment, once weekly during the treatment period and finally
on the day of sacrifice. The amount of food and water intake was
recorded daily and expressed as an average for 7 days. Animals
were treated orally once a day using the metal oropharyngeal
cannula for a duration of 28 days [15].
On the 29th day from commencement of treatment, all animals
were terminated for the evaluation of other signs of toxicity. To this
effect, they were starved for 24hours, then anesthetized using an
overdose of ethyl-ether. The thoracic region was rapidly dissected
and blood samples collected through cardiac puncture. Part of it
was collected in heparinized test-tubes, whereas the other part
was collected in heparin-free test-tubes which allowed coagulation
and the subsequent collection of serum. The animals were then
sacrificed through cervical dislocation and selected organs including
the heart, kidney, liver and spleen isolated. They were freed from all
connective tissue moisture, examined for morphological changes
such as the presence of any kind of lesions and then weighed
using an electronic balance (NVT 1601/1, OHAUS, USA). Both
blood samples were preserved at 4oC±1 for hematological and
biochemical tests, respectively. As regards hematological tests,
red blood cell (RBC), white blood cell (WBC) and platelet numbers
as well as the percentage lymphocytes, monocytes, eosinophils
and neutrophils were determined using the fully automated
hematology analyzer (URIT3300) (Prasanth et al. 2014). The serum
was processed for biochemical parameters including creatinine,
alanine aminotransferase (ALT) (serum glutamate pyruvate
transaminase, SGPT), aspartate aminotransferase (AST) (serum
glutamic oxaloacetic transaminase, SGOT), alkaline phosphatase
(ALP), albumin, total cholesterol and triglycerides [16,17].
Ethical Consideration
Animals were handled in accordance with the Organization for Economic Cooperation and Development (OECD) guidelines for testing chemicals 423 and 425 (OECD, 2008a&b) and the experimental protocol was approved by the University of Buea Institutional Animal Care and Use Committee (UB-IACUC).
Statistical Analyses
Values were expressed as Mean±standard error of mean (SEM). Mean values were calculated for each animal and quantitative comparisons between groups established from those means. One way ANOVA followed by Duncan test was used to analyse the data with the aid of the SPSS for windows version 20.0 software. Significant levels were tested at p<0.05.
Results
Acute Toxicity
Single oral administration of the leaf-aqueous extract of P. arboreus at 2000 mg/kg and 5000 mg/kg produced no behavioral changes, no signs of toxicity and no mortality in rats after treatment. Since there were no clinical signs of toxicity and death in the tested doses, LD50 value of the extract was found to be greater than 5000mg/kg.
Sub-Acute Toxicity
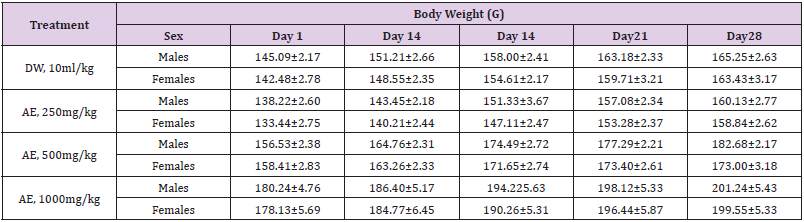
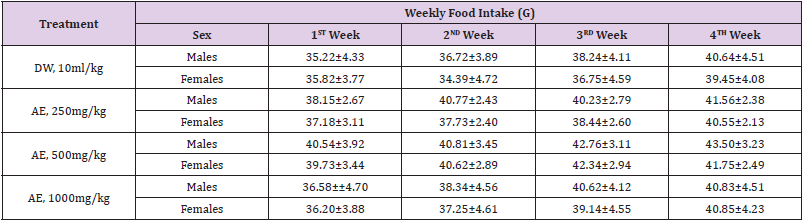
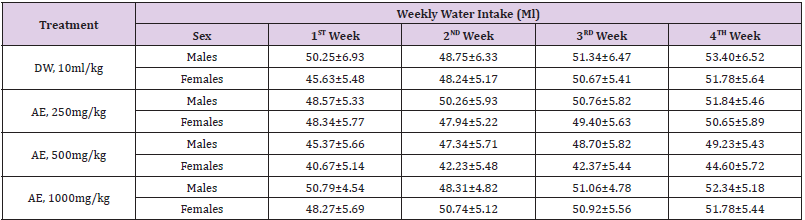
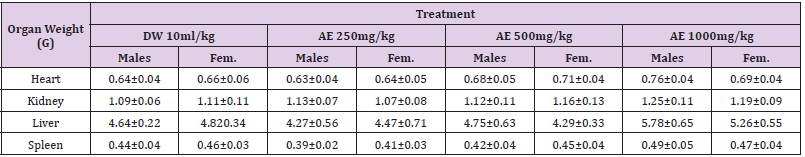
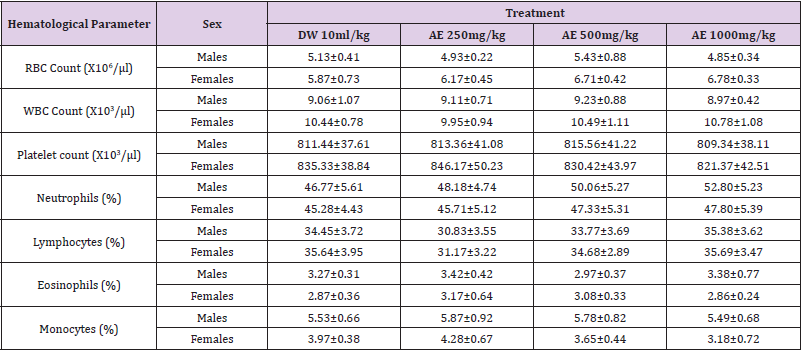
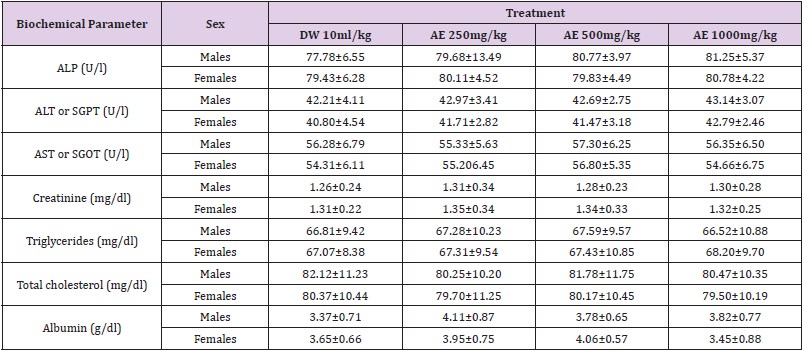
Following 28 days repeated oral administration of the leafaqueous extract of P. arboreus at 250, 500 and 1000mg/kg doses to rats, there were no treatment related toxicity signs and mortality observed in both sexes of rats treated. No significant (p<0.05) differences in weekly body weight gain were observed between the extract-treated and control rats Table 1. As presented in Table 2, repeated treatment of rats with the leaf-aqueous extract of P. arboreus produced a non-significant (p<0.05) difference in the weekly food intake of the animals, compared to their control counterparts. Like in food intake, similar results were obtained in water intake of animals following 28days repeated treatment with the leaf-aqueous extract of P. arboreus, compared to the control animals Table 3. Sub-acute treatment of rats with the leaf-aqueous extract of P. arboreus did not produce any significant (p<0.05) difference in the weight of organs such as the heart, kidney, liver and spleen, compared to the control animals Table 4. Results of the effects of sub-acute treatment of animals with the leaf-aqueous extract of P. arboreus on hematological parameters are presented in Table 5. According to the table, there were no statistically significant (p<0.05) differences in the hematological parameters measured between the control and extract-treated groups. Subacute administration of the leaf-aqueous extract of P. arboreus did not show any significant changes in biochemical parameters such as alkaline phosphatase (ALP), alanine amino transferase (ALT), aspartate aminotransferase (AST), creatinine, triglycerides, total cholesterol and albumin when compared to control group Table 6.
Table 1: Effects of the Sub-acute (28 days) administration of the leaf-aqueous extract of P. arboreus on the body weight of rats.
Values: Mean±SEM; DW: distilled water; AE: aqueous extract
Table 2: Effects of sub-acute (28days) administration of the leaf-aqueous extract of P. arboreus on weekly food intake (g) in rats.
Values: Mean±SEM; DW: distilled water; AE: aqueous extract; Fem.: females.
Table 3: Effects of sub-acute (28days) administration of the leaf-aqueous extract of P. arboreus on weekly water intake (ml) in rats.
Values: Mean±SEM; DW: distilled water; AE: aqueous extract.
Table 4: Effects of sub-acute (28days) administration of the leaf-aqueous extract of arboreus on the weight of some selected organs.
Values: Mean±SEM; DW: distilled water; AE: aqueous extract.
Table 5: Effects of sub-acute (28days) administration of the leaf-aqueous extract of P. arboreus on hematological parameters of rats.
Values: Mean±SEM; AE: aqueous extract; DW: distilled water %: percentage; μl: microliters.
Table 6: Effects of sub-acute (28days) administration of the leaf-aqueous extract of P. arboreus on biochemical parameters.
Values: Mean±SEM; AE: aqueous extract; ALP: alkaline phosphatase; ALT: alanine aminotransferase; AST: aspartate aminotransferase; DW: distilled water; SGOT: serum glutamic oxaloacetic transaminase: SGPT: serum glutamic pyruvic transaminase.
Discussion
In developing countries, herbal medicines have become famous
in healthcare, and some have been falsely considered as safe without
understanding the possible health effects and thus commonly
used as self-medication [18]. As the use of plant-based products
increases, it is important to screen the toxicological profile of these
plants to confirm the safety and efficacy of those natural sources.
Though P. arboreus is popular in the Bayang folk medicine as a
sex enhancer, there is lack of data on its toxicological profile and
adverse effects. Therefore, toxicity studies were necessary not only
to identify the further range of doses in animal studies, but also to
explain the probable clinical signs evoked by its extracts. Hence
the present experiment was undertaken to evaluate the possible
effects of the short term and long-term administration of its leafaqueous
extract. Throughout the 14 days of observation period, no
morbidity or mortality was observed in the extract-treated rats.
In the present study, the results showed no adverse events in the dose groups 2000 mg/kg and 5000 mg/kg which indicate that the
LD50 was greater than 5000 mg/kg. According to the OECD [12], a
substance that does not cause mortality at the limit dose of 5000
mg/kg would have a DL50 greater than this limit dose and can
be considered non-toxic. When treatment related toxicity is not
identified in acute toxicity, sub-acute toxicity is assessed to ensure
safety after repeated exposure over a relatively long period of time.
In the repeated dose (28-day) oral toxicity study, there were
neither deaths nor treatment-related signs observed in all the
groups of animals. After exposure to a few possible toxic substances,
there will be changes in body weight gain and internal organ weights
which would reflect toxicity [19]. The body weight changes are
markers of adverse effects of drugs and chemicals and if the body
weight loss occurred is more than 10% of the initial body weight
it will be considered as statistically significant [20,19]. There were
no significant differences in body weight gain of both control and
treated groups. We can therefore deduce that leaf-aqueous extract
of P. arboreus is almost non-toxic. Organ weight also is an important
indicator of physiological and pathological status of animals. The
relative organ weight is fundamental to confirm whether the organ
was exposed to the injury or not. The heart, kidney, liver and spleen
are the primary organs affected by metabolic reactions caused by
toxicants [21]. In the present study, organ weights in all the treated
groups of both sexes were not significantly different from those of
control groups. Hence, it can equally be concluded that leaf-aqueous
extract of P. arboreus is almost non-toxic.
Since proper food and water intake is necessary to the
physiological status of the animal and to the achievement of
a better response to test substance under investigation, these
parameters were measured in our study [17,22]. Our findings
revealed that both food and water consumption were not affected
by the administration of the extract. Thus, this indicates that there
was no interruption in the metabolism of carbohydrate, protein
and fat. Analysis of blood parameters is important in the evaluation
of risks associated with test compounds under investigation as
the changes in the hematological system have a greater indicative
value for human toxicity, when the data are converted from animal
studies [18]. Repeated treatment of animals with the aqueous
extract of P. arboreus for 28 days did not produce any changes in
hematological parameters including RBC, WBC and platelet counts
as well as the percentage lymphocytes, monocytes, eosinophils
and neutrophils, an indication that the extract did not affect the
blood cellular components or their production. Transaminases
such as serum glutamic oxaloacetic transaminase (SGOT) and
serum glutamic pyruvic transaminase (SGPT) (also called aspartate
aminotransferase [AST] and alanine aminotransferase [ALT],
respectively) are normally present in the heart and liver and their
release into blood indicates heart or liver damage.
They are therefore well-known good indicators of liver and
heart function and are used as biomarkers to predict the probable
toxicity of drugs and xenobiotics [23,24]. Normally, destruction to
the liver parenchymal cells will result in an increase of both these
enzymes in the blood [25]. Interestingly, there were no changes
in the ALT and AST levels in our investigations, which reveal
that the extract did not affect the liver function/ or metabolism.
Furthermore, determination of plasma proteins like albumin is
required in order to assess the synthetic capacity of the liver and
decrease in plasma proteins therefore tend to reflect chronic
damage [26]; hence, the no alteration in the level of albumin in
extract-treated animals is another indication that their liver was
not affected. The extract did not equally provoke any change in the
serum levels of total cholesterol and triglycerides. The action of the
extract in maintaining a stable serum lipid profile could be through
the induction of the inhibition of reductase hydroxyl methyl glutanyl
CoA (HMG-CoA) leading to reduction of hepatic synthesis and
intestinal absorption of cholesterol. Indeed, the inhibition of HMGCoA
by flavonoids which have been identified in our plant extract
has been reported. This effect of the leaf-aqueous extract of P.
arboreus on lipid profile could suggest its beneficial effects against
lipid peroxidation and subsequently on oxidative stress [27]. The
reduction of blood lipids is an efficient method to prevent and treat
cardiovascular affections [28]. This could explain its empirical use
in treating hypertension, since arterial hypertension is generally
associated to dyslipidemia [29].
Conclusion
Results of our findings indicate that treatment with single oral doses of 2000 mg/kg and 5000 mg/kg of the plant extract did not result in any toxic signs or mortality in the acute toxicity studies; likewise, daily oral administration of the extract of P. arboreus for a period of 28 days did not cause mortality, changes in body weight and body weight gain. Hematological and biochemical examinations proved that the extract is safe. Hence, the no-observed adverseeffect level (NOAEL) of the extract was found to exceed 1000 mg/ kg/day. Overall, it can be concluded that the leaf-aqueous extract of P. arboreus is well tolerated.
For more
Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.