Coexistence of Vagus Nerve Stimulation and Epicardial Implantable Cardioverter-Defibrillator System, Possible Interference: A Case Report and Systematic Review of the Literature
Introduction
Vagus nerve stimulation (VNS) is an effective treatment for drug-resistant epilepsies in adults [1] and children [2]. Although VNS is generally well tolerated, rare cases of severe bradycardia and asystole related to vagal stimulation are described [3,4]. The coexistence of VNS with an electronic cardiac implantable device, i.e., pacemaker [5-7] or implantable cardioverter-defibrillator (ICD) [8], in patients suffering from both refractory epilepsy and arrhythmias are rarely reported, none in pediatric age or in a patient with epilepsy and congenital heart disease. The presence of both an implantable neurostimulator and an implantable cardiac device in the same patient raises the concern that stimulation from VNS therapy systems would be detected by the cardiac device, leading to inappropriate delivery of therapy. In this review, we report the first case in literature of a child with a VNS device and a ICD system implanted via an epicardial approach, and summarize the current literature available on concomitant therapy with both VNS and implantable electronic cardiac system, with a focus on possible interference between the devices.
Case Report
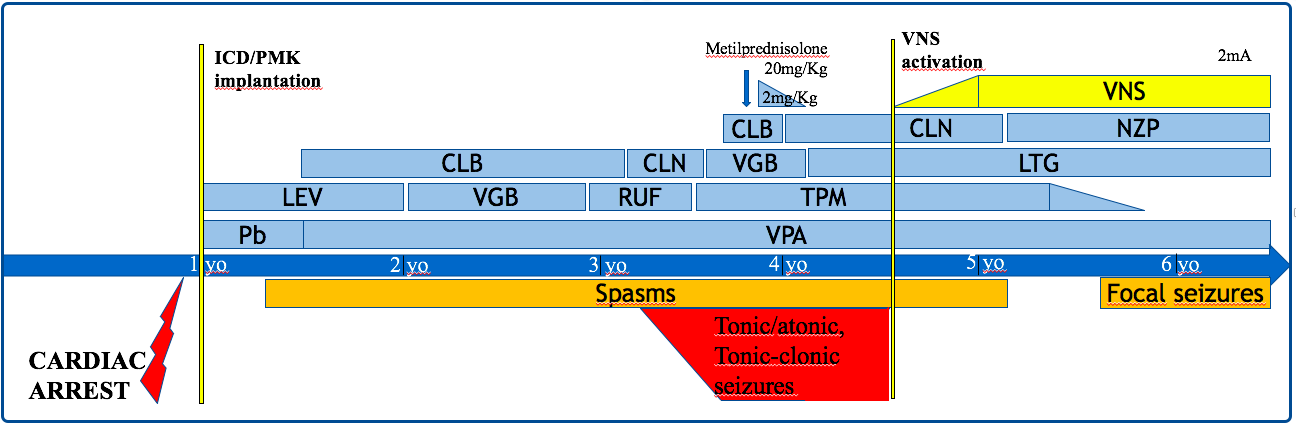
A 7-year-old child was born at term after uneventful pregnancy. At the age of 9 months, she was diagnosed with long QT syndrome [mean corrected QT interval (QTc), 546 ms] and hypertrophic cardiomyopathy; treatment with propranolol was immediately started. Genetic testing for KCNQ1, KCNH2, SCN5A, KCNE1 and KCNE2, as well as CGH-array, resulted negative. At the age of 11 months, the patient has survived a cardiac arrest due to ventricular fibrillation after prolonged resuscitation maneuvers, resulting in a diffuse ischemic encephalopathy with basal ganglia involvement and asymmetrical distribution for left hemisphere predominant damage. At 12 months of age, an ICD (Protecta XT 354 DR, Medtronic, Inc.) with epicardial leads placement (i.e., a bipolar pacesense lead placed on the right ventricle, and a Transvene® 6937A transvenous superior vena cava coil, Medtronic, Inc., placed in the transverse sinus as the defibrillator coil) was implanted; the pocket was seated below the left costal margin. Prophylactic treatment with Phenobarbital was started. Few days after discharge, she was re-admitted for repetitive focal seizures, and levetiracetam (LEV) was added to Phenobarbital. At 17 months of age, she presented with right hemiparesis, focal seizures and developmental delay. During the follow-up, the epilepsy has proven to be highly drug resistant: valproate, clobazam, vigabatrin, rufinamide, topiramate, clonazepam and lamotrigine were alternatively added in therapy with poor effect. At 3 years of age, she began to experience recurrent multifocal clonic seizures (about 2-3/week), that caused the child to fall. Resective/disconnective surgery was excluded due to the multifocality of recoded seizures and extensiveness of the cerebral damage.
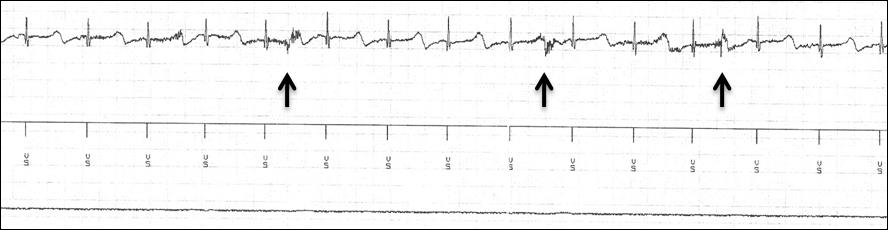
Vagus nerve stimulation was considered the best option and the implantation (Livanova 103 and Cyberonics 404) was performed on the left thoracic side (the can was located between the left chest and the upper abdomen, not far from the ICD) (age 4.5 years old). The timeline of clinical events and treatments is represented in (Figure 1). About 1 month after VNS implantation, concern was raised just before the activation of intermittent vagal stimulation. Any possible interference between the VNS and ICD was carefully detected through continuous ECG and ICD programmer monitoring. The ventricular sensing mode was found configured using the tip-to-coil bipole. In order to minimize the recording of external interference, the true bipolar electrode was set as the ventricular detection mode. First, the impedance of the VNS was tested for few seconds: 3 electrical noise signals were observed on the far-field recording (i.e. can/coil channel), but not detected by the ICD on the sensing channel (i.e. true RV bipole), with all the artifacts that fell during the ventricular repolarization phase (Figure 2). Second, the VNS was activated at 0.25 mA and afterwards increased at 0.50 mA, noticing a high frequency signal only at the beginning of each step to increase the output, but again without abnormal events in the marker channel (Figure 3A). Finally, the VNS was tested at the highest output; the same phenomenon that occurred at lower outputs was observed, without any inappropriate event.
Figure 2: Impedance test during the first activation of the VNS and electrograms recording from ICD (from bottom to top, respectively: can/coil channel, reference marks, true bipolar RV lead channel). The arrows indicate high frequency noise right at the beginning of the impedance test.
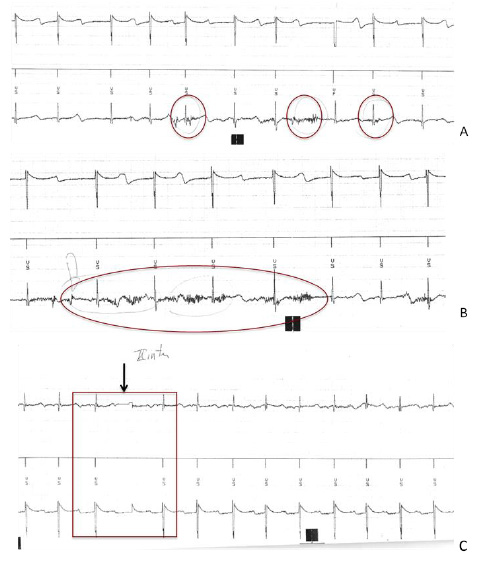
Figure 3: Vagal nerve stimulation device programming and ICD monitoring.
A. Electrograms from ICD (from bottom to top: can/coil channel, reference marks, true bipolar RV lead channel) while a
current of 0.5 mA is delivered from the VNS: noise are traced by the can/coil channel but not from the true bipolar one nor
from the reference one.
B. Electrograms (as in figure A) during VNS with 1.25 mA and coughing. Noise is documented on the can/coil channel but not
on the others, all under ICD sensing threshold.
C. Electrograms (from bottom to top: true bipolar RV channel, references marks, can/coil channel). At the maximum output
(black arrow), a sharp signal on the first and third channel without any mark on the reference channel was documented. This
episode may be related to a sort of isolated reset of ICD recording due to electromagnetic interference, as well as to a loss of
telemetry just for the initial increase in energy.
It was concluded that the use of both devices simultaneously was safe due to the absence of pacing and sensing interferences, and the VNS was programmed at 0.50 mA as the starting output current. Correct QT interval at that time was 490 ms (under the maximum tolerated dose of beta-blockers drugs). Over time, all parameters of VNS, i.e. output current, frequency, pulse width, and stimulation on/off times (duty cycle), were adjusted to reach the maximum tolerable output current. To test the neurological effect of the VNS, a stepwise approach was performed to gradually increase the intensity of vagal stimulation; a new ICD monitoring was performed each time the VNS current was increased. A month later, the VNS output was increased to 1-1.25 mA. When the VNS was activated at these values, the child started coughing, and noise was concurrently observed on the far-field recording, but again without any noise recorded in the near-field electrogram, as well as without any abnormal events recorded in the ICD marker channel (Figure 3B). At this time, the QTc was slightly longer, 510 ms. A 24h ECG Holter was performed to evaluate arrhythmia occurrence and QT modification: no arrhythmia occurred and QTc varied from 499 to 566 ms during the recording. An improvement in seizures was gradually observed as the VNS intensity was progressively tuned up. During a control 6 months later, an odd phenomenon has been observed on the programmer when the energy was increased, i.e., a transient lack of recordings and marking, but with a clear electrical input (Figure 3C). This episode may be related to a sort of isolated reset of ICD recording due to electromagnetic interference, as well as to a loss of telemetry just for the initial increase in energy. In the subsequent 2 years of follow-up, the child has never experienced seizures, and a marked improvement in alertness, motor and cognitive performances and in the electroencephalogramme findings were noticed. The VNS activation by a magnet to provide on-demand stimulation to prevent or shorten a seizure was regularly used for clusters of spasm, often with apparent advantage and without interference. No further significant QTc prolongation was observed during progressive optimization of VNS stimulation.
Discussion
In the 1990s, with the success of several early clinical trials, VNS was approved for the treatment of refractory epilepsy, and later for refractory depression. It exerts antiepileptic or antiepileptogenic effect possibly through neuromodulation of certain monoamine pathways and vagal afferent pathways, resulting in alterations of seizure-generating regions.9 Beyond epilepsy, VNS is also under investigation for the treatment of chronic heart failure, inflammation, asthma, and pain [9].
Vagus Nerve Stimulation for Epilepsy Treatment and Cardiac Effects
Vagal efferent pathways innervating the heart is known to induce inhibition of sinoatrial node activity resulting in decreased heart rate, atrioventricular conduction, and excitability of the His- Purkinje system. However, the effects of VNS on cardiovascular autonomic tone of patients with refractory epilepsy remain poorly understood. In the one hand, cardiac changes related to VNS in epileptic patients have been reported to be rare, and data on heart rate variability, baroreflex sensibility, and blood pressure monitoring revealed only slight alterations of the autonomic cardiac tone with no clinically and hemodynamically relevant effects [10,11]; on the other hand, several cases of bradyarrhythmia during implantation have been reported [12,13], and pilot studies showed higher vagal tone [14] and lower heart rate in patients with VNS [15]. Intraoperatively, bradycardia and asystole can occur, albeit rarely, during lead impedance testing. This could be due to either collateral current spread or inadvertent placement of electrodes on vagus nerve cardiac branches. In these cases the procedure was immediately terminated and the device removed. Although rare, delayed arrhythmias and syncope have been reported after long-term use [16]. Iriarte and coll. describe a case of late asystole in a patient whose VNS had been implanted 9 years before the arrhythmia onset; each run of stimulation produced bradyarrhythmias and very often severe asystolia due to atriumventricular block.
The Authors hypothesized a possible influence of the status epilepticus; it is a well-known phenomenon that vegetative changes are frequent during epileptic seizures, and these changes may have predisposed the patient to VNS-related cardiac arrhythmia. Therefore, these data suggest that new-onset episodes of nonepileptic origin in patients with VNS merit urgent cardiac evaluation.Furthermore, in patients with intermittent vagal stimulation, temporary imbalances of cardiac autonomic activity due to increased stimulation of the vagus nerve could be expected to paradoxically modify the QT interval and increase the arrhythmic risk [17]. Desimone et coll. reported a transient QT interval prolongation and a potentially increased arrhythmic risk in long QT syndrome early after left sympathetic denervation [18]. However, studies have not found an excess of cases of sudden death in patients with VNS [19], but VNS should be contraindicated in patients with type 3 Long QT Syndrome, in whom ventricular tachyarrhythmias are triggered by increased vagal tone. In our case, no bradycardia or arrhythmias or dramatic QT modification were noticed acutely and in the follow-up.
Coexistence of Vagus Nerve Stimulation for Refractory Epilepsy and Pacemaker/Implantable Cardioverter Defibrillator: Possible Interference
The presence of both an implantable neurostimulator and an implantable cardiac device raises the concern that stimulation from VNS therapy systems would be detected by the implantable cardiac device, leading to inappropriate delivery of therapy. Interference between the two devices could lead to high-frequency VNS being detected by the cardiac device, triggering a change in cardiac pacing or a inappropriate delivery of a high-voltage shock. However, the possible interaction of VNS and cardiac devices such as pacemaker and ICD is poorly known. Only three patients with refractory epilepsy implanted with pacemaker and VNS [5-7], and only one with ICD and VNS [8] are reported in literature; in all these cases the implantation of the cardiac device was performed using a transvenous approach. Cáceres, et al. [5] reports the case of a 45-year-old woman with a VNS and pacemaker implanted for AV block with asystole secondary to seizures and without a cardiovascular disease. Yun [6] published the case of a 55-year-old woman who was implanted with a pacemaker after VNS-induced bradycardia; no information is given about follow-up. Beal [7] describes the case of a 17-year-old boy who was implanted with pacemaker for seizure induced bradycardia; VNS was subsequently implanted but removed just several weeks later for pocket infection. The only report of ICD and VNS implantation concerns a patient (56-year-old) with a bipolar disorder and a poorly defined cardiac disease (“syncope and ventricular tachyarrhythmias”) [8]. To our knowledge, this is the first report of a patient with VNS system and epicardial defibrillator lead placement, which also describes apparent interference between VNS and ICD, nevertheless without compromising the correct function of both devices; moreover, this is the first case of ICD and VNS coexistence reported in childhood and in a patient with severe congenital heart disease, for whom careful and long-lasting ICD monitoring is also available.
In our case, the epicardial approach for ICD implantation was preferred to the transvenous placement due to the young age of the patient. Drug resistant epylepsia was the indication to VNS therapy. Based on clinical effect and tolerability, it was planned to pursue an approach that was expected to start with low output (0.25-0.75 mA) and gradually increase the VNS amplitude to compensate the tissue resistance [9]. At the time of the first VNS activation, noise was noticed on ICD can/coil channel during the impedance test and at the beginning of each increase in the stimulation output up to the maximum power. However, the noise was only evident on the far-field channel, but it was never identifiable in the narrowfield channel, either because it was not recorded or it was under the threshold of sensing and therefore discarded by the ICD. At follow-up, inappropriate ICD events were never noticed, allowing the girl to effectively control seizures and to be safely protected by the ICD. Nevertheless, there are few possible concerns to be considered when such electrical devices co-exist. The activation of the VNS generates an electrical current that can be detected by the ICD; in our case, high frequency signals were only recorded on the far-field channel, while the true bipolar channel (i.e. right ventricular sensing channel) was always free from interference, or alternatively it was only affected by signals under the threshold of sensing. But it could be speculated that a critical threshold could be exceeded when the energy reaches a high output, causing detection of inappropriate electrical signals and possible ICD malfunction. Therefore, to be sure to avoid sensing the output of the VNS on the ICD, the VNS must be tested at maximum output and the sensitivity of the pacemaker must be varied to assess for interaction. With the highest VNS energy output, an odd behavior was recorded with a sort of “black out” of the sensing/pacing function.
A possible explanation could be an isolated loss of telemetry between the programmer and the ICD, due to electromagnetic interference from the high energy delivered by the VNS. In the less likely hypothesis of a transient ICD malfunction due to a VNS interference, a loss of pacing could not be detected since the patient had spontaneous ventricular rhythm, but concerns would arise in pacemaker-dependent patients.A further consideration concerns the implantation site of VNS and ICD, which could influence their mutual functioning. In the childhood, the small size of the patient limited the space to be shared by the 2 devices. In our case, the devices were placed close to each other in the upper abdomen, theoretically overlapping the ICD sensing/shock field and the VNS electrical exit and potentially interfering with correct recognition and treatment of arrhythmias. Moreover, the epicardial location of the ICD leads could also facilitate possible interference due to their position outside the heart, being anatomically closer to the VNS can and the efferent vagal nerve endings. However, no interference was detected in our patient at follow up.
Vagus Nerve Stimulation for Heart Failure Treatment
Heart failure is characterized by an overactive sympathetic nervous system and parasympathetic withdrawal, and this autonomic imbalance contributes to the progression of the disease. As such, modulation of autonomic nervous system by device-based therapy has been speculated an attractive treatment target. Recent trials have published data on heart failure patients implanted with a cervical VNS system with lead placement on either the right or left cervical vagus nerve and given chronic stimulation for up to 12 months; in some of these studies, VNS activation and inactivation periods were unrelated to the cardiac cycle (i.e. open loop) [20-22], while a right ventricular sensing lead was used for a closed loop system in the INOVATE-HF trial [23]. However, no significant benefit on mortality, cardiac remodeling and heart failure hospitalization has been demonstrated, albeit a small benefit in functional capacity has been shown. A total of 441 patients enrolled in these studies, most of which (364/441 patients) in the INOVATE-HF trial [23], had both an implantable VNS system and an implantable cardiac device, and none interference or interaction between the two systems has been reported.
Conclusion
Vagus nerve stimulation has proven to be an effective treatment for refractory epilepsy. The positioning of both a VNS system and a pacemaker/ICD in the same patient appears to be safe, as supported by reports of patients affected by epilepsy and by studies on heart failure as well as by the case of the patient reported above. Cardiac device monitoring during VNS tapering should be performed. To be sure to avoid sensing the output of the VNS on the pacemaker/ ICD, it is critical that the VNS is tested at maximum output and the sensitivity of the cardiac device has varied to assess for interaction. Furthermore, the case reported by our Institute suggests that the coexistence of VNS with epicardial ICD is feasible and can be safe also in children.
For more Articles on : https://biomedres01.blogspot.com/



No comments:
Post a Comment
Note: Only a member of this blog may post a comment.