Gum Arabic Improves Reproductive Function Associated with Alteration of Testicular Visfatin mRNA Expression in Alloxan Induced Diabetic Rats
Introduction
Bioactive natural principles are reported used as a source of
medicinal agents for several decades [1,2]. Herbal-based principles
play vital roles in the treatment the prevention of diabetes mellitus
(DM) [3,4]. In developing countries, herbal products considered
as a traditional medicine for meliorating diabetes complications
[5,6]. Approximately, fifty millions of couples worldwide are
experience infertility problems [7,8], mainly through comparative
contributions in change of lifestyle [9] and metabolic disorder
factors [10]. Herbal bioactive agents are principally used
worldwide to improve reproductive capacity [11]. Various types
of medicinal plants are reported to be used in mixture with
assisted reproductive technologies [11,12] to decrease the cost
[13,14] and boost infertility success rate [15]. DM is a group of
metabolic disorders that characterized by hyperglycemia [16] due
to the defects in secretion of insulin, insulin action, or both [17].
The chronic hyperglycemia in diabetes patients is reported to be
associated with long-term tissue damage [18], dysfunction [18], and
failure of various organs [19], in particular the reproductive organs
[20]. The prolonged hyperglycemia is associated with protracted
dysfunction [21], damage, and collapse of functioning of a variety of
organs, including kidneys [22], and blood vessels [23], nerves [24],
and testis [25,26]. Hyperglycaemia trigger reactive oxygen species
production [27], which in turn causes cell damage via different
mechanisms [28-30]. Both tissues and cellular damage ultimately
consequences in the secondary complications of DM [31].
Many experimental and clinical reports have been conducted on the molecular mechanisms responsible for the changes induced by DM in reproductive system of male but much remains to be clarified [32]. Some studies implicated that the diabetes induced male infertility through histological damage of the epididymis [33], decreased sperm motility [34], semen volume [35], sperm counts, motility and morphology [36] and disruption of seminiferous tubular morphology [37]. Moreover, DM induced male infertility via decreasing serum levels of luteinizing hormone (LH), follicular stimulating (FSH) [38] and testosterone [39]. Visfatin, a newly is adipocyte hormone that discovered, that has strong relationship with type 2 diabetic patients [40]. Visfatin is found to bind to the insulin receptor at a site different from that of insulin causes hypoglycaemia [41] by reducing glucose release from liver cells [42] and stimulating glucose utilization in adipocytes [43] and myocytes [44]. Visfatin is upregulated by hypoxia in adipocytes [45], inflammation and hyperglycemia and downregulated by insulin, somatostatin and statins. Previous studies reported that plasma levels and tissue expression of visfatin increased in parallel with diabetes [46]. Visfatin was reported to have insulin-mimetic effects [47] and lowers plasma glucose levels [48]. In addition, high glucose concentrations obviously increased visfatin production in cultured adipocytes [49]. Moreover, blood visfatin levels were found to increase in parallel with hyperglycemia [46]. Moreover, it is reported that type 2 diabetic patients found to have elevated plasma visfatin concentrations [50]. On the other hand, plasma visfatin concentrations were reported to be decreased in diabetic women during gestational period [51]. Visfatin plasma levels together with its expression were found to be associated with obesity [52], insulin resistance [53] and type 2 diabetic patients [54].
Gum Arabic (GA) from Acacia seyal and Acacia senegal, is dried sticky exudate that contains soluble dietary fiber [55]. It is commonly used in food industry [56] and pharmaceutical preparations [57] as preservative and emulsifier [58]. Several studies confirmed that GA lowers blood cholesterol [59], improves antioxidant capacity [60] decreases blood glucose [61], decreases blood pressure in patients with type 2 DM patients [62]. However, the effects of GA on Visfatin in testis of type I diabetic rats have not been reported. In addition, it is less clear whether GA may modify testosterone levels in type I diabetic rats. Therefore, in the present study, we induced type I diabetic in rat to elucidate our hypothesis that the treatment of GA to type I diabetic rat may change blood Visfatin and its mRNA and protein in the testis of diabetic rat and the changes in Visfatin may correlate with blood glucose, testosterone concentrations, testis and body weight in diabetic rat.
Materials and Methods
Animals and Experimental Design
Male Sprague-Dawley (SD) rats weighing 200±10g were purchased Sudanese National Center for Research and housed in a controlled environment with a 12h light–dark cycle. Animals were acclimatized for one week before the study and had free access to water and standard rat chow throughout the experimental period. The rats were divided into 3 groups: control group (n=20) given standard animal pellet and water ad libitum; diabetic group (n=20); and diabetic group (n=20) given 10% Gum Arabic in drinking water for 8 weeks. Type I diabetes mellitus was induced as described by Adeyi, et al. [63]. Briefly, Alloxan monohydrate was purchased from Sigma-Aldrich China (Shanghai, China), and type I diabetes mellitus was induced by single intraperitoneal injection of 150mg/kg of Alloxan monohydrate dissolved in normal saline after an overnight fast. Surviving rats after 3 days with blood glucose concentration more than 200mg/dl of blood were considered as Alloxan-induced diabetic rats and used as type I diabetic models rat for further study. All rats were euthanized after 8 weeks of treatment. On day 70, the rats were fasted overnight, urine and blood samples were collected prior to euthanasia. Body weights and organ weights were recorded; blood and tissue samples were collected and stored at -80 °C until analyzed. The experiment procedures were approved by the Animal Ethics Committee of University of Nyala.
Sperm Analysis
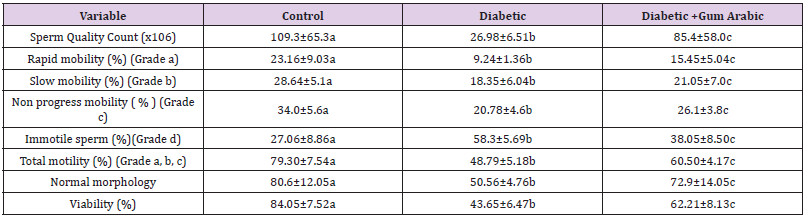
Testes with epididymis were removed, and the caudal epididymidis were separated from the testis and the semen was collected. Squeezed semen was incubated in buffer containing BSA at 37 °C for 30 minutes. The normal morphology of sperm, motility, sperm count and its viability were measured in groups of experimental rats. We used Makler Chamber and light microscopy (Olympus Co., Tokyo, Japan) for sperm movement analysis. The motility was expressed as the percentages of progressive motility including rapid (Grade a) and slow (Grade b) spermatozoa, nonprogressive (Grade c) and immotile (Grade d) spermatozoa. The morphology of the spermatozoa was evaluated using the original dilution for motility, diluted 1:20 with 10% neutral buffered formalin. The sperms were classified according to the presence of one or more abnormal features such as tail defect (colloid, irregular, Short, or multiple tails); neck and middle piece (bend middle piece, distended irregular, abnormality thin middle piece); and head defects (small or large size, round head, double or detached head). The data were presented as percentage of morphological normal sperm.
Blood Glucose
Serum was obtained from blood by centrifugation (at 3000rpm for 15min) and stored at -20 ºC until analyzed. Blood glucose was measured using assay kits (Nanjing Jiancheng Bioengineering Company, Nanjing, China), according to the manufacturers’ instructions.
Plasma Testosterone
After decapitation of rats, blood was collected from the ruptured cervical vessels in new heparinized tubes for plasma testosterone measurement. The plasma was obtained after centrifugation (2,400rpm, 20min, 3.5 °C) in a refrigerated device and frozen at -20 °C until measurement of hormone levels. Plasma testosterone concentrations were measured by radioimmunoassay method, using the Testosterone Maia® kit (Biochem Immuno System) according to the manufacturers’ instructions. All plasma samples were dosed in the same assay, to avoid inter-assay errors. The lower detection limit for testosterone was 0.064ng/mL, with a 4% intraassay error.
Real-Time PCR and Gene Expression
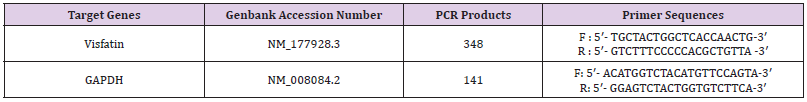
About 100mg of liver was ground in liquid N2, and a portion of about 50mg was used for RNA extraction using TRIzol total RNA kit (Invitrogen, Biotechnology Co, Ltd, Carlsbad, CA, USA) according to the manufacturer’s instruction. Two approaches were taken to ensure that all the total RNA preparations are free of genomic DNA contamination. Firstly, total RNAs were treated with 10 U DNase I (RNase Free, D2215, Takara, Japan) for 30 min at 37 °C, and purified according to the manufacturer’s protocol. Secondly, the primers for the reference gene (GAPDH) were designed to span an intron, so any genomic DNA contamination could be reported easily with an extra product in the melting curves for real-time PCR. Real-time PCR was performed in Mx3000P (Stratagene, USA) according to our previous publications [64]. Mock RT and No Template Controls (NTC) were included to monitor the possible contamination of genomic and environmental DNA at both RT and PCR steps. The pooled sample was made by mixing equal quantity of RT products (cDNA) from all samples and was used for optimizing the PCR condition and tailoring the standard curves for each target gene. The melting curves were performed to insure a single specific PCR product for each gene. The PCR products were sequenced to validate the identity of the amplicons. Primers specific for Visfatin (Table 2) was synthesized by Geneary (Shanghai, China). Rat GAPDH was used as a reference gene for normalization purpose. The method of 2-ΔΔCt was used to analyze the real-time PCR data [65]. The mRNA abundances were presented as the fold change relative to the average level of the control group.
Protein Extraction and Western Blot Analysis
Protein extracts from 50mg of frozen liver tissue were prepared as described in our previous publication [66]. The protein concentrations were measured by Pierce BCA Protein Assay Kit (Thermo Scientific, USA). Western blot analysis for Visfatin (Cayman Chemical Company, USA, diluted 1:200) and Visfatin (sc- 20176, Santa Cruz Biotechnology, USA, diluted 1:200) were carried out according to the recommended protocols that offered by the company. Β-actin (Cayman Chemical Company, USA, and diluted 1:10.000) was used as a reference in the Western blot analysis.
Histopathology Examinations
Livers were fixed in paraformaldehyde solution and embedded in paraffin, sectioned serially at 4lm and stained with hematoxylin and eosin (H&E) to investigate the morphologic changes in control, diabetic and diabetic rats treated with Gum Arabic. Slides at every time-point were stained with H&E and observed under a light microscope (Nikon, Tokyo, Japan).
Statistical Analysis
Descriptive statistics analysis was made to check the normality and homogeneity of variances before parametric analyses. Body weight, organs weight, semen parameters, blood glucose, plasma testosterone, relative quantitative data of gene expression, in addition to western blot analysis data were statistically analyzed by one-way ANOVA using IBM SPSS statistics 21.0 for Windows, followed by a least-significant difference (LSD) test for individual comparisons. A P-value ≤0.05 was considered significant.
Results
Effect of GA Treatment on Food Intake, Body Weight and Organs Weight
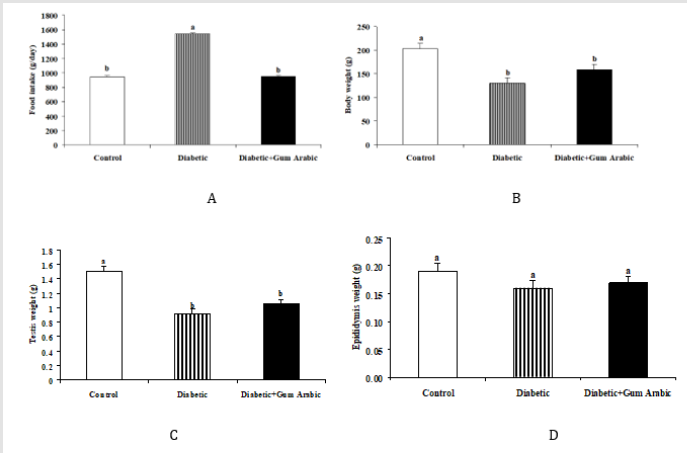
In the present study, Alloxan induced diabetic rat groups showed significantly increases in food intake compared to the control group. The treatment of GA significantly (P< 0.05) decreased food intake (Figure 1A). No significant differences were observed final body weight (Figure 1B), testis weight (Figure 1C) and epididymis weight (Figure 1D) regarding the treatment of GA.
Blood Glucose, Serum Testosterone and Visfatin Concentrations
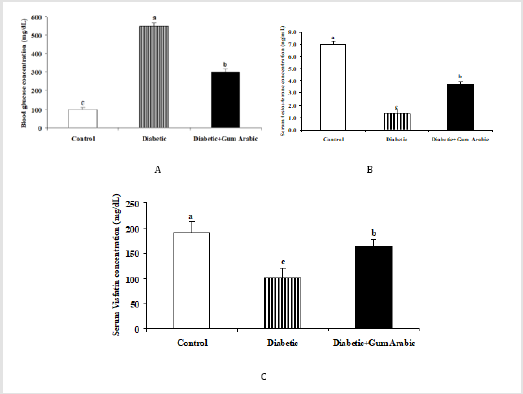
Treatment of GA significantly (P<0.05) decreased blood glucose concentrations compared to the control and diabetic groups (Figure 2A). In contrast, the treatment of GA significantly (P<0.05) increased serum testosterone concentration compared to the diabetic rat group (Figure 2B). Likewise, the treatment of GA significantly increased serum Visfatin concentration compared to the diabetic rat group (Figure 2C).
Parameters Correlations
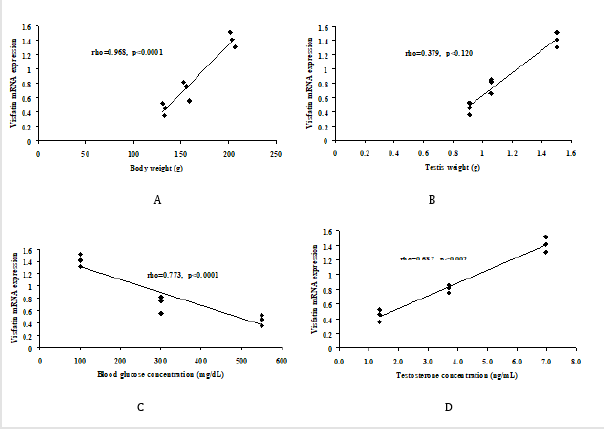
The present study, expression of Visfatin mRNA revealed a positive correlation with body weight (Figure 3A), testis weight (Figure 3B), and serum testosterone concentration (Figure 3D). However, the expression of Visfatin showed negative correlation with blood glucose concentrations (Figure 3C).
Effect of GA Treatment on Semen Quality
Alloxan induced diabetic rat group showed significant decreases in sperm count, sperm rapid mobility, slow motility, non-progress mobility, total motility and sperm vitality compared to the control. However, the treatment of GA significantly (P < 0.05) improved the above mentioned sperm quality parameters. On the other hand, diabetic rat group showed significant increases in immotile sperm compared to the control group. But the treatment of GA significantly (P < 0.05) reduced immotile sperm compared to that in diabetic rat (Table 1).
Testicular Visfatin mRNA Expression
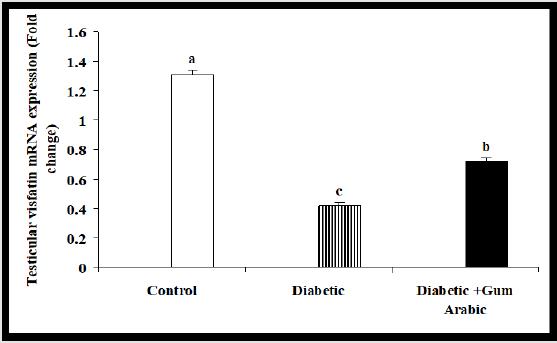
In the current study, the treatment of Alloxan significantly reduced testicular mRNA expression of Visfatin compared to the control group. But the treatment of GA significantly (P<0.05) upregulated testicular Visfatin mRNA expression compared to the diabetic rat group (Figure 4A).
Testicular Visfatin Protein Expression
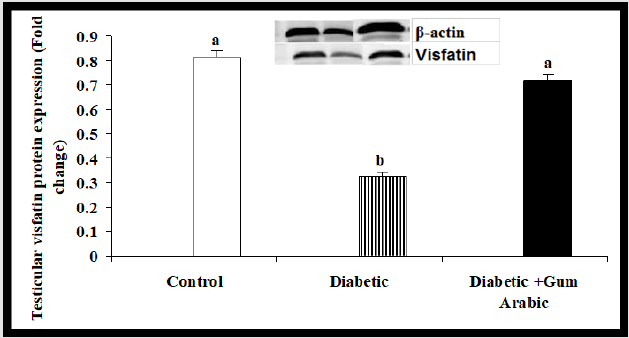
In the present study, Alloxan treated group rats significantly showed the reduction of testicular Visfatin protein expression compared to the control group. However, the treatment of GA significantly (P<0.05) increased testicular Visfatin protein expression compared to the diabetic rat group (Figure 5A).
Histopathological Features
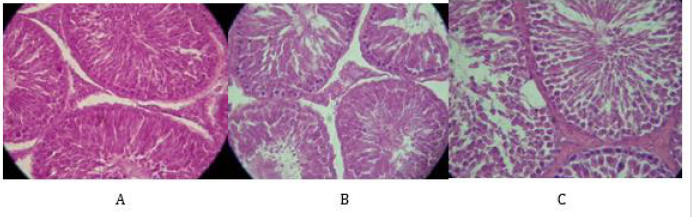
Histological features in testis of the control group revealed normal cytology of the testis with no visible degenerative changes (Figure 6A). The testis of diabetic rats showed obvious degenerative changes with vacoulations (Figure 6B). However, the treatment of GA slightly protected the testis of diabetic rats from degenerative damages compared to control and diabetic rats (Figure 6C).
Discussion
Diabetes mellitus (DM) is a potential pandemic metabolic disorder causes dysfunction of reproductive performance [67]. In the present study, the treatment of Gum Arabic (GA) decreased food intake associated with decreases in body weight and testis weight. These findings are in line with previous studies that the treatment of dietary fibre decreased food intake and decreased body weight in mice [67-69]. The decreases in food intake or body weight by GA could be due to the fact that a number of studies revealed that the dietary fiber enhanced satiation and satiety [70], changed the glycaemic index [71], influenced the gastric emptying, and secretion of gastric hormone [72] thus, it reduces body weight [67]. In addition, the supplementation of GA in the form of drinking water decreased blood glucose. These findings are consistent with our earlier studies that GA decreased blood glucose both in normal and diabetic rat [73,74] or normal mice [61]. It is well documented that the consumption of GA inhibits absorption of glucose in the intestine through interaction of membrane abundance in sodium-glucose transporter 1(SGLT1) in mice [75]. However, the mechanism of underlying the reduction of blood glucose by GA is not yet fully elucidated, due to the lack of researches in this field. Several experimental studies documented that the induction of diabetes in animal models has impaired reproductive performance via decreasing testicular function thus caused male fertility [76]. In the present study, the treatment of GA increased sperm quality parameters in diabetic rat. These results confirm our previous studies which showed the improvement of semen quality by GA in diabetic rat model [77]. In addition, it was report that ginger (Zingiber officinale) a dietary fibre enhanced reproductive capacity via increasing semen quantity in diabetic rat [78]. Moreover, the histological changes of the testis showed marked degeneration in the testis of Alloxan-induced diabetic rats. However, the treatment of GA significantly protected the testis of diabetic rats from oxidative damage. These findings are agreed with those reported by that the GA supplementation protected various tissues including nephrotoxicity [79, 80], hepatotoxicity [81] and testis [77] in rat.
Visfatin, one of the adipokines [82], is present in a variety of tissues including the testis [83]. In the present study, the treatment of GA decreased blood glucose concentrations whereas, increased serum testosterone concentrations. Our findings are in line with previous studies that GA treatment decreased glucose concentration and production in rats [60,61]. In addition, lowfat intervention together with high-fiber increased testosterone levels after in human [84]. Conversely, intervention of low fat diet simultaneously with high fiber diet decreased serum and urine androgens in men [85]. In addition, treatment of GA significantly (P<0.05) increased testicular Visfatin mRNA expression associated with upregulation of Visfatin protein expression compared to the diabetic group. Testis of diabetic rats showed obvious degeneration whereas; slight degeneration was seen in GA treated rats compared to control. Visfatin mRNA expression revealed a positive correlation with body, testis weight and serum testosterone concentrations, while a negative correlation was observed with blood glucose concentrations.
Conclusion
Our findings conclude that GA may improve reproductive
performance and it may be useful to meliorate the diabetic
infertility complications in male.
For more Articles on : https://biomedres01.blogspot.com/


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.